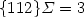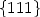Research Article
Theoretical approach of the characterization of gradientsin elastic properties by acoustic microscopy
-
- Published online by Cambridge University Press:
- 15 March 1999, pp. 221-226
-
- Article
- Export citation
Rapid Communication
A special study on the sensitivity of electron mobility with respect to the tunnel oxide thickness for a floating gate electron-tunneling mosfet
-
- Published online by Cambridge University Press:
- 15 January 1999, pp. 1-2
-
- Article
- Export citation
Emission of carbon clusters from sooting plasma
-
- Published online by Cambridge University Press:
- 15 February 1999, pp. 111-114
-
- Article
- Export citation
Research Article
Write and erase mechanism of surface controlled bistable nematic pixel
-
- Published online by Cambridge University Press:
- 15 March 1999, pp. 227-230
-
- Article
- Export citation
Suppression of the “notch effect” in microwave surface resistance in YBa2Cu3O7 films on MgO (100) substrate by deposition of ultra-thin SrTiO3 seed layers
-
- Published online by Cambridge University Press:
- 15 January 1999, pp. 3-8
-
- Article
- Export citation
On a phase transformation produced by mechanical activation in iron pyrite
-
- Published online by Cambridge University Press:
- 15 February 1999, pp. 115-121
-
- Article
- Export citation
Correlated electronic and magnetic peculiarities ofLa2NiO4+δ : Charge and spin ordering*
-
- Published online by Cambridge University Press:
- 15 February 1999, pp. 123-126
-
- Article
- Export citation
On the relation between Frank-Read source natureand fine slip line structure
-
- Published online by Cambridge University Press:
- 15 March 1999, pp. 231-236
-
- Article
- Export citation
Determination of the characteristic interfacial electronicstates of {111} Cu-MgO interfaces by ELNES
-
- Published online by Cambridge University Press:
- 15 January 1999, pp. 9-18
-
- Article
- Export citation
Ultrasonic attenuation spectroscopy and light scattering study of the ageing of very fine emulsions
-
- Published online by Cambridge University Press:
- 15 February 1999, pp. 127-134
-
- Article
- Export citation
Determination of the electron mean free path in the 1–1.8 eV energy range inthin gold layers using ballistic electron emission microscopy
-
- Published online by Cambridge University Press:
- 15 March 1999, pp. 237-242
-
- Article
- Export citation
Liquid metal embrittlement: A state-of-the-art appraisal
-
- Published online by Cambridge University Press:
- 15 January 1999, pp. 19-31
-
- Article
- Export citation
What is a physical measure of spatial inhomogeneity comparable to the mathematical approach?
-
- Published online by Cambridge University Press:
- 15 March 1999, pp. 243-249
-
- Article
- Export citation
Phase transitions in mixed disordered crystals Rb1−x Kx CaF3 (0 < x < 1) investigated by Raman spectroscopy
-
- Published online by Cambridge University Press:
- 15 January 1999, pp. 33-44
-
- Article
- Export citation
Atomic structure of {112} Σ = 3 twin boundary in β–SiC
-
- Published online by Cambridge University Press:
- 15 February 1999, pp. 135-141
-
- Article
- Export citation
Production of carbon nanotubes by the solar route
-
- Published online by Cambridge University Press:
- 15 March 1999, pp. 251-256
-
- Article
- Export citation
Sculptured thin film Šolc filters for optical sensingof gas concentration
-
- Published online by Cambridge University Press:
- 15 January 1999, pp. 45-50
-
- Article
- Export citation
Backscattering of electrons from selected oxides:MgO, SiO2, and Al2O3
-
- Published online by Cambridge University Press:
- 15 February 1999, pp. 143-148
-
- Article
- Export citation
A predictive sampling scale model for direct torque control of the induction machine fed by multilevelvoltage-source inverters
-
- Published online by Cambridge University Press:
- 15 January 1999, pp. 51-61
-
- Article
- Export citation
Determination of the bond strength of some microns coatings using the laser shock technique
-
- Published online by Cambridge University Press:
- 15 February 1999, pp. 149-153
-
- Article
- Export citation