Magnesium-chlorophoenicite may be differentiated from the Mn-analogue chlorophoenicite, because 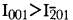 for magnesium-chlorophoenicite at 7Å, whereas
for magnesium-chlorophoenicite at 7Å, whereas 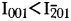 for chlorophoenicite.
for chlorophoenicite.
In a review of the literature for the Mineral Powder Diffraction File by Bayliss et al. (1980), powder X-ray diffraction data could not be found of the mineral species magnesium-chlorophoenicite, (Mg,Mn)3Zn2(AsO4)(OH,O)6. Dunn (1981) states that the powder X-ray diffraction data of magnesium-chlorophoenicite is essentially identical to that of chlorophoenicite (Mn analogue) and confirms that the minerals are isostructural.
With the crystal structure parameters determined by Moore (1968) for a Harvard University specimen from New Jersey of chlorophoenicite, a powder X-ray diffraction pattern was calculated with the programme of Langhof, Physikalische Chemie Institute, Darmstadt. The calculated pattern was used to correct and complete the indexing of the powder X-ray diffraction data of chlorophoenicite specimen ROM M15667 from Franklin, Sussex County, New Jersey, U.S.A. by the Royal Ontario Museum (PDF 25-1159). With the correctly indexed data of ROM M15667, the unitcell parameters were refined by least-squares analysis and are listed in Table 1.
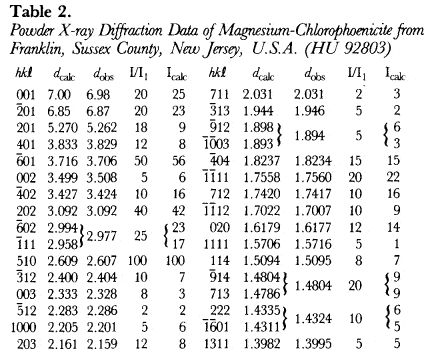
The most magnesium-rich magnesium-chlorophoenicite found in the literature is a description of Harvard University specimen 92803 from Franklin, Sussex County, New Jersey, U.S.A. by Dunn (1981), where Mg is slightly greater than Mn. A 114.6 mm Debye-Schemer film taken of HU92803 with Cu radiation and a Ni filter (CuKα = 1.5418Å) was obtained from Dr. P. Dunn and measured visually. The unit-cell parameters, which were refined by least-squares analysis starting from the unit-cell parameters of PDF 25-1159 in space group C2/m(#12), are listed in Table 1, and give F28 = 4.1(0.050,136) by the method of Smith & Snyder (1979).
The hkl, dcalulated, dobserved and relative intensities (I/I1) of HU92803 are presented in Table 2. With the atomic positions and temperature factors of chlorophoenicite determined by Moore (1968), the Mn atomic positions occupied by 50% Mg and 50% Mn, and the unit-cell parameters of HU92803, a powder X-ray diffraction pattern was calculated and Icalculated is recorded in Table 2. A third powder X-ray diffraction pattern was calculated with the Mn atomic positions fully occupied by Mg. Because the atomic scattering factor of Mn is more than twice greater than Mg, chlorophoenicite may be differentiated from magnesium-chlorophoenicite based upon the calculated intensities of the first three reflections given in Table 3.
Although the a, c and β unit-cell parameters of chlorphoenicite are similar to those of magnesium-chlorphoenicite, the b unit-cell parameter of chlorophoenicite is significantly greater than that of magnesium-chlorophoenicite (Table 1). The b unit-cell parameter represents the 0–0 distance of the Mn octahedra (Moore, 1968). Since the size of Mn is greater than that of Mg, chlorophoenicite may be differentiated from magnesium-chlorophoenicite based upon the b unit-cell parameter given in Table 1.
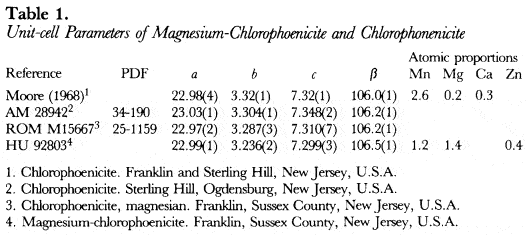
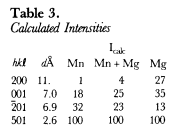
American Museum of Natural History (New York, N.Y., U.S.A.) specimen 28942 from Sterling Hill, Ogdensburg, New Jersey is composed of willemite, haidingerite and magnesian chlorophoenicite. A spectrographic analysis of the magnesian chlorophoenicite shows As, Mg, Mn and Zn. Powder X-ray diffraction data (PDF 34-190) of the magnesian chlorophoenicite was collected by diffractometer with Cu radiation and a graphite 0002 monochromator (Kα1 = 1.5405) at a scanning speed of 0.125° 2θ per minute. The unit-cell parameters, which were refined by leastsquares analysis starting from the unit-cell parameters of PDF 25-1159, are given in Table 1. Specimen AM 28942 is called chlorophoenicite, because of its large b unit-cell parameter (Table 1), and the I/I1 of 25 for reflection 001 and of 50 for reflection 201 compared to the Icalculated in Table 3.