Symposium C – Structure and Properties of Interfaces in Ceramics
Research Article
The Morphology of Cu Clusters on SrTiO3(001) at Initial Stages of Metal Film Growth
-
- Published online by Cambridge University Press:
- 15 February 2011, 3
-
- Article
- Export citation
In-Situ Electron Microscopy Studies of The Behavior of Metal Particles on Ceramic Substrates
-
- Published online by Cambridge University Press:
- 15 February 2011, 11
-
- Article
- Export citation
Interaction of Pt Films With ZnO(0001) and ZnO(0001)
-
- Published online by Cambridge University Press:
- 15 February 2011, 17
-
- Article
- Export citation
Effect of Surface Structure on The Adsorption of CO(II) on ∝-Al2O3: A Glancing Angle Xafs Study
-
- Published online by Cambridge University Press:
- 15 February 2011, 23
-
- Article
- Export citation
Cesium/Oxide Interactions for Ultrathin Films on ∝-Al2O3(0001) and ∝-Al2O3(1102)
-
- Published online by Cambridge University Press:
- 15 February 2011, 29
-
- Article
- Export citation
XPS Studies at the Interface of Ti/AIN Ceramic
-
- Published online by Cambridge University Press:
- 15 February 2011, 35
-
- Article
- Export citation
Growth and Structure of Al/MgO Interfaces
-
- Published online by Cambridge University Press:
- 15 February 2011, 41
-
- Article
- Export citation
Chemical Bonding, Structure, and Morphology of Mg/∝-Al2O3 and MGO / ∝-Al2O3 Interfaces
-
- Published online by Cambridge University Press:
- 15 February 2011, 47
-
- Article
- Export citation
High Resolution Transmission Electron Microscopy of Metallic Film/Laser-Irradiated Alumina Couples.
-
- Published online by Cambridge University Press:
- 15 February 2011, 53
-
- Article
- Export citation
Auger Electron Spectroscopy of Metallic Film/Laser-Irradiated Alumina Couples
-
- Published online by Cambridge University Press:
- 15 February 2011, 59
-
- Article
- Export citation
Atomic Bonding at Oxide Surfaces
-
- Published online by Cambridge University Press:
- 15 February 2011, 67
-
- Article
- Export citation
The Atomic-Scale Characterization of Defects on Cleaved Vanadium and Molybdenum Oxide Surfaces Using Stm
-
- Published online by Cambridge University Press:
- 15 February 2011, 79
-
- Article
- Export citation
The Influence of Surface Structure on the Interaction of Water with TiO2 (100)
-
- Published online by Cambridge University Press:
- 15 February 2011, 91
-
- Article
- Export citation
The Adsorption of Liquid and Vapor Water on TiO2 (110) Surfaces: The Role of Defects
-
- Published online by Cambridge University Press:
- 15 February 2011, 97
-
- Article
- Export citation
Structures of Bonding Interface by Forming Hydrogen Bridges
-
- Published online by Cambridge University Press:
- 15 February 2011, 103
-
- Article
- Export citation
A Study of the Interface Between SnF2 Particles and Air in Stannous Fluoride Heated in Air
-
- Published online by Cambridge University Press:
- 15 February 2011, 109
-
- Article
- Export citation
Direct Determination of Grain Boundary Atomic Structure in SrTiO3
-
- Published online by Cambridge University Press:
- 15 February 2011, 115
-
- Article
- Export citation
Dynamic Behavior of Twins In BaTiO3
-
- Published online by Cambridge University Press:
- 15 February 2011, 121
-
- Article
- Export citation
High Resolution Electron Microscopy and Computer Simulation Studies of the Atomic Structure of Tilt Boundaries in TiO2
-
- Published online by Cambridge University Press:
- 15 February 2011, 127
-
- Article
- Export citation
The Interaction of Twin Boundaries with Grain Boundaries in YBa2Cu3O7-δ
-
- Published online by Cambridge University Press:
- 15 February 2011, 133
-
- Article
- Export citation

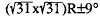 reconstructed surface yields a value of 0.27. Thermal desorption mass spectrometry (TDMS) shows that surface reconstruction eliminates the high binding energy states of Cs (2.7 eV/atom), consistent with the observed changes in adsorption probability. In contrast, reconstruction of the (1102) surface produces only minor changes in Cs adsorption. Xray photoelectron spectroscopy (XPS) indicates that no formal reductive/oxidative chemistry takes place at the interface. We interpret the facile adsorption and strong binding of Cs on sapphire to result from Cs interacting with coordinatively unsaturated oxygen.
reconstructed surface yields a value of 0.27. Thermal desorption mass spectrometry (TDMS) shows that surface reconstruction eliminates the high binding energy states of Cs (2.7 eV/atom), consistent with the observed changes in adsorption probability. In contrast, reconstruction of the (1102) surface produces only minor changes in Cs adsorption. Xray photoelectron spectroscopy (XPS) indicates that no formal reductive/oxidative chemistry takes place at the interface. We interpret the facile adsorption and strong binding of Cs on sapphire to result from Cs interacting with coordinatively unsaturated oxygen.