Here is reported a new scheme to accurately determine the vapor pressure of
undercooled, liquid, and high temperature solid materials. The method relies on an imaging
technique that measures the time variation of the radius of an electrostatically levitated
sample. This scheme, compared to other techniques, offers unique opportunity to explore not
only the liquid above the melting point but also the undercooled states of highly reactive
materials in a contamination free environment. This was exemplified in this paper with
titanium. For the first time, we report the vapor pressure $(V_{\rm p})$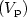 of its liquid phase over a large temperature range, covering the undercooled region. Over the 1700 to 2050 K temperature range, it was measured as Log $V_{\rm p}(T) = 9.154 - 17978\ T^{-1}$
of its liquid phase over a large temperature range, covering the undercooled region. Over the 1700 to 2050 K temperature range, it was measured as Log $V_{\rm p}(T) = 9.154 - 17978\ T^{-1}$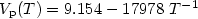 (3%). Similarly, for high temperature solid titanium, the vapor pressure could be expressed as Log $V_{\rm p}(T) = 16.634 - 32960\ T^{-1}$
(3%). Similarly, for high temperature solid titanium, the vapor pressure could be expressed as Log $V_{\rm p}(T) = 16.634 - 32960\ T^{-1}$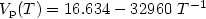 (6%) over the 1770 to 1940 K temperature interval. From these data, the average latent heats of vaporization and sublimation were calculated respectively as 344.8 kJ/kg (8%) and 632.1 kJ/kg (6%) respectively.
(6%) over the 1770 to 1940 K temperature interval. From these data, the average latent heats of vaporization and sublimation were calculated respectively as 344.8 kJ/kg (8%) and 632.1 kJ/kg (6%) respectively.