Recombinant retroviruses are a commonly used gene delivery tool in human gene therapy clinical trials as they permanently integrate the therapeutic DNA into the genome of the target cell. Despite their widespread use, however, retroviral gene transfer efficiencies remain disappointingly low. In the years since their initial development as vectors, it has become clear that the physicochemical properties of the recombinant retrovirus, as well as the properties of the target tissue, have a major impact on the success of gene transfer. In particular, the interaction between the native negative charge present on the vector and target cell membranes results in a significant electrostatic barrier which must be overcome in order for the transduction process to begin. Using a recently developed retrovirus adsorption assay, we have demonstrated that this electrostatic interaction is the dominant interaction during the initial steps of transduction. We have also established that cationic polymers enhance adsorption, and ostensibly transduction, by neutralizing this electrostatic barrier. Interestingly, we also found that this enhanced adsorption could be demonstrated even in the absence of a cellular receptor for the virus, suggesting that the first step of transduction does not involve binding of the virus to currently identified receptors, but is either a non-specific adsorption process or involves binding to an, as of yet, uncharacterized class of receptor. Based upon our finding that virus adsorption is non-gp70 mediated, we propose a new physical model of this initial step of transduction which is diffusion-limited, receptor and envelope independent, and modulable by positively charged compounds. Our findings may have significant implications for other gene and drug delivery systems which interact directly with target tissues at the level of the cell membrane.

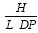 Where H = %molar level of unreacted L-lysine units, L = %molar level of substituted palmitoyl units and DP = square root of the degree of polymerisation of the polymer. Probe sonication of an aqueous dispersion of PLP samples resulted in the production of stable nanoparticles (80 -170nm in diameter) as imaged by electron microscopy. Nanoparticles were able to encapsulate the hydrophilic fluorophore fluorescein isothiocyanate (FITC)-dextran and encapsulation increased as the level of unreacted lysine terminal amino groups in PLP increased thus increasing as the level of hydrophilic domains increased. The size of both the nanoparticles and the vesicles was directly influenced by the molecular weight of PLP. PLPs of molecular weight 32,000 – 48,000 and 89,000 – 140,000 resulted in nanoparticles of 85 – 114 nm and 125 – 167 nm in diameter respectively and PLP of molecular weight 25,000 and 89,000 gave rise to polymeric vesicles of 252 nm and 570 nm in diameter respectively.
Where H = %molar level of unreacted L-lysine units, L = %molar level of substituted palmitoyl units and DP = square root of the degree of polymerisation of the polymer. Probe sonication of an aqueous dispersion of PLP samples resulted in the production of stable nanoparticles (80 -170nm in diameter) as imaged by electron microscopy. Nanoparticles were able to encapsulate the hydrophilic fluorophore fluorescein isothiocyanate (FITC)-dextran and encapsulation increased as the level of unreacted lysine terminal amino groups in PLP increased thus increasing as the level of hydrophilic domains increased. The size of both the nanoparticles and the vesicles was directly influenced by the molecular weight of PLP. PLPs of molecular weight 32,000 – 48,000 and 89,000 – 140,000 resulted in nanoparticles of 85 – 114 nm and 125 – 167 nm in diameter respectively and PLP of molecular weight 25,000 and 89,000 gave rise to polymeric vesicles of 252 nm and 570 nm in diameter respectively.