No CrossRef data available.
Published online by Cambridge University Press: 15 March 2011
Nanoparticles and polymeric vesicles for drug delivery and other industrial applications have been prepared by the probe sonication of poly-L-lysine graft copolymer amphiphiles in aqueous media. The amphiphiles, which have a poly-L-lysine backbone and varied levels of both hydrophilic methoxypolyethylene glycol (Mw ∼ 5,000) and hydrophobic palmitoyl pendant groups, were prepared from 2 different molecular weight poly-L-lysine hydrobromide samples (Mw ∼4,000 and ∼20,000 respectively). Poly-L-lysine based amphiphilic polymers (PLPs) were characterised using light scattering, 1H NMR and an assay for the level of free amino groups. Steric factors appear to limit the final level of lysine group modification that can be achieved and even an excess amount of grafting reactants still resulted in the production of polymers in which 22 – 26 mole% of the lysine terminal amino groups remain unsubstituted. Polymeric unilamellar vesicles (220 – 570nm in diameter) imaged by electron microscopy were produced by probe sonication of PLP, cholesterol. Vesicle formation was possible over a narrow spectrum of polymer architecture and was favoured by a low molecular weight and a low level of palmitoyl substitution. A vesicle formation index (F') has thus been derived for this PLP, cholesterol system = 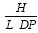 Where H = %molar level of unreacted L-lysine units, L = %molar level of substituted palmitoyl units and DP = square root of the degree of polymerisation of the polymer. Probe sonication of an aqueous dispersion of PLP samples resulted in the production of stable nanoparticles (80 -170nm in diameter) as imaged by electron microscopy. Nanoparticles were able to encapsulate the hydrophilic fluorophore fluorescein isothiocyanate (FITC)-dextran and encapsulation increased as the level of unreacted lysine terminal amino groups in PLP increased thus increasing as the level of hydrophilic domains increased. The size of both the nanoparticles and the vesicles was directly influenced by the molecular weight of PLP. PLPs of molecular weight 32,000 – 48,000 and 89,000 – 140,000 resulted in nanoparticles of 85 – 114 nm and 125 – 167 nm in diameter respectively and PLP of molecular weight 25,000 and 89,000 gave rise to polymeric vesicles of 252 nm and 570 nm in diameter respectively.
Where H = %molar level of unreacted L-lysine units, L = %molar level of substituted palmitoyl units and DP = square root of the degree of polymerisation of the polymer. Probe sonication of an aqueous dispersion of PLP samples resulted in the production of stable nanoparticles (80 -170nm in diameter) as imaged by electron microscopy. Nanoparticles were able to encapsulate the hydrophilic fluorophore fluorescein isothiocyanate (FITC)-dextran and encapsulation increased as the level of unreacted lysine terminal amino groups in PLP increased thus increasing as the level of hydrophilic domains increased. The size of both the nanoparticles and the vesicles was directly influenced by the molecular weight of PLP. PLPs of molecular weight 32,000 – 48,000 and 89,000 – 140,000 resulted in nanoparticles of 85 – 114 nm and 125 – 167 nm in diameter respectively and PLP of molecular weight 25,000 and 89,000 gave rise to polymeric vesicles of 252 nm and 570 nm in diameter respectively.