Invited Feature Papers
Articles
Research Article
Heat transport through interfaces with and without misfit dislocation arrays
-
- Published online by Cambridge University Press:
- 15 October 2012, pp. 2718-2723
-
- Article
- Export citation
Reviews
Hydrogen in oxide semiconductors
-
- Published online by Cambridge University Press:
- 18 May 2012, pp. 2190-2198
-
- Article
- Export citation
Invited Feature Paper
Transforming large-scale industrially produced carbon nanotubes to high-performance electrode materials for lithium-ion batteries
-
- Published online by Cambridge University Press:
- 13 December 2011, pp. 410-416
-
- Article
- Export citation
Reviews
Reactive magnetron sputtering of transparent conductive oxide thin films: Role of energetic particle (ion) bombardment
-
- Published online by Cambridge University Press:
- 08 February 2012, pp. 765-779
-
- Article
- Export citation
Invited Feature Paper
Analysis of resistance switching and conductive filaments inside Cu-Ge-S using in situ transmission electron microscopy
-
- Published online by Cambridge University Press:
- 31 January 2012, pp. 886-896
-
- Article
- Export citation
Articles
Strain relaxation defects in perovskite oxide superlattices
-
- Published online by Cambridge University Press:
- 19 March 2012, pp. 1436-1444
-
- Article
- Export citation
Synthesis and characterization of poly(vinylidene fluoride)/carbon nanotube composite piezoelectric powders
-
- Published online by Cambridge University Press:
- 27 July 2012, pp. 2352-2359
-
- Article
- Export citation
Effects of different sulfurization conditions on the characterization of CuIn(SxSe1−x)2 thin films
-
- Published online by Cambridge University Press:
- 14 March 2012, pp. 1112-1116
-
- Article
- Export citation
Formation and properties of strontium-based bulk metallic glasses with ultralow glass transition temperature
-
- Published online by Cambridge University Press:
- 05 July 2012, pp. 2593-2600
-
- Article
- Export citation
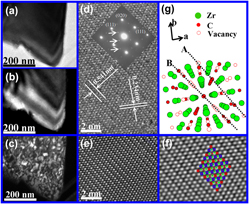
Superstructural nanodomains of ordered carbon vacancies in nonstoichiometric ZrC0.61
-
- Published online by Cambridge University Press:
- 21 March 2012, pp. 1230-1236
-
- Article
- Export citation
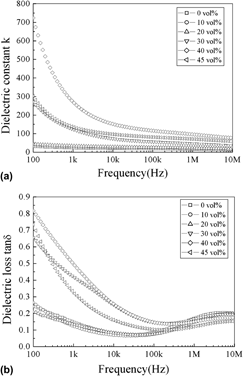
Microstructure and dielectric behavior of the three-phase Ag@SiO2/BaTiO3/PVDF composites with high permittivity
-
- Published online by Cambridge University Press:
- 29 February 2012, pp. 991-998
-
- Article
- Export citation
Determination of shear creep compliance of linear viscoelastic solids by instrumented indentation when the contact area has a single maximum
-
- Published online by Cambridge University Press:
- 09 May 2012, pp. 1565-1572
-
- Article
- Export citation
Shear strength and sliding behavior of Ni/Al2O3 interfaces: A first-principle study
-
- Published online by Cambridge University Press:
- 23 March 2012, pp. 1237-1244
-
- Article
- Export citation
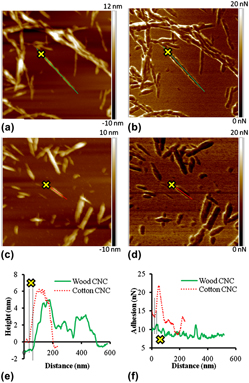
Size effects on the nanomechanical properties of cellulose I nanocrystals
-
- Published online by Cambridge University Press:
- 23 September 2011, pp. 528-536
-
- Article
- Export citation
Invited Feature Papers
Articles
Research Article
Application of small-scale testing for investigation of ion-beam-irradiated materials
-
- Published online by Cambridge University Press:
- 10 October 2012, pp. 2724-2736
-
- Article
- Export citation
Articles
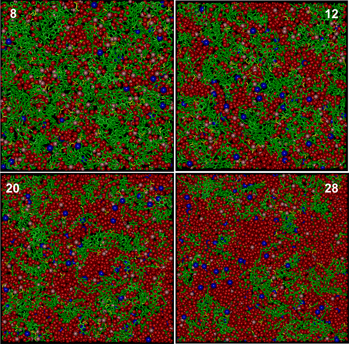
Molecular modeling of the morphology and transport properties of two direct methanol fuel cell membranes: Phenylated sulfonated poly(ether ether ketone ketone) versus Nafion
-
- Published online by Cambridge University Press:
- 07 June 2012, pp. 1927-1938
-
- Article
- Export citation
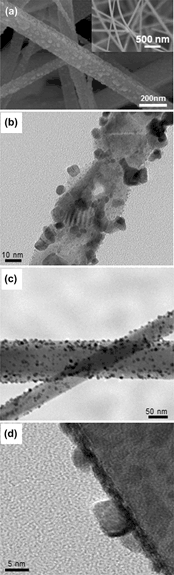
Platinum nanoparticle-functionalized tin dioxide nanowires via radiolysis and their sensing capability
-
- Published online by Cambridge University Press:
- 24 May 2012, pp. 1688-1694
-
- Article
- Export citation
Li+ for Na+ ion-exchange-induced phase separation in borosilicate glass
-
- Published online by Cambridge University Press:
- 28 February 2012, pp. 999-1005
-
- Article
- Export citation
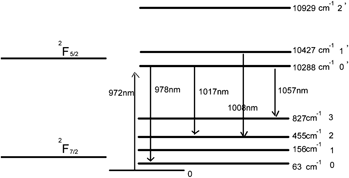
The spectroscopic investigation of ZnWO4: Yb3+ single crystal
-
- Published online by Cambridge University Press:
- 10 May 2012, pp. 2096-2100
-
- Article
- Export citation
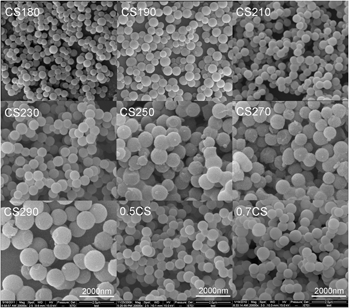
Control of the morphology and chemical properties of carbon spheres prepared from glucose by a hydrothermal method
-
- Published online by Cambridge University Press:
- 02 February 2012, pp. 1117-1123
-
- Article
- Export citation