Article contents
Transforming large-scale industrially produced carbon nanotubes to high-performance electrode materials for lithium-ion batteries
Published online by Cambridge University Press: 13 December 2011
Abstract
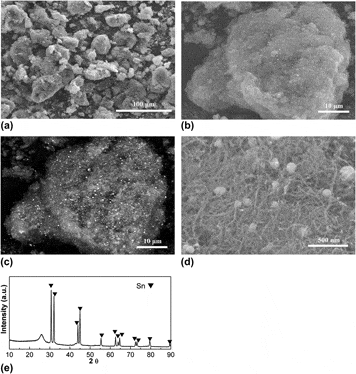
Large-scale industrial production of carbon nanotubes (CNTs) has recently become available, but there are relatively few reports of the investigation of these industrially produced bulk CNTs as potential electrode materials for electrochemical energy storage such as lithium-ion batteries (LIBs). Here, we report our evaluation of the electrochemical performance of the industrially produced CNTs from one manufacturer and our utilization of a kinetically controlled, vapor diffusion synthesis method combined with in-situ carbothermal reduction to homogeneously grow nanocrystalline tin (Sn) particles (∼200 nm) in the matrix of the CNTs, yielding a Sn@CNTs composite. After surface coating with a layer of carbon coating (3–4 nm), this composite was transformed to a surface-modified Sn@CNTs composite that exhibited much higher reversible capacity, initial Coulombic efficiency, and rate capacity than the pristine CNTs as anode materials for LIB.
Keywords
- Type
- Invited Feature Paper
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 3
- Cited by


