Introduction
Species-focused conservation aims to prevent extinction by designing and implementing actions for local and global protection (Hoffmann et al., Reference Hoffmann, Hilton-Taylor, Angulo, Böhm, Brooks and Butchart2010; Hoban & Vernesi, Reference Hoban and Vernesi2012). To design effective conservation actions, conservationists often rely on population monitoring (Nichols & Williams, Reference Nichols and Williams2006; Clutton-Brock & Sheldon, Reference Clutton-Brock and Sheldon2010). Monitoring provides information and estimates of population size and growth (i.e. vital rates such as survivorship, migration rate and fecundity), which can be used to assess the viability of the population over the long or short term (Johnson et al., Reference Johnson, Mills, Stephenson and Wehausen2010; Ali et al., Reference Ali, Kauffman, Amin, Kibara, King and Mallon2018; Lacy, Reference Lacy2019). In addition, monitoring underpins the implementation of management strategies to promote the recovery of threatened species (McCarthy et al., Reference McCarthy, Possingham, Day and Tyre2001; Yoccoz et al., Reference Yoccoz, Nichols and Boulinier2001; Lindenmayer & Likens, Reference Lindenmayer and Likens2010b).
The mountain bongo Tragelaphus eurycerus isaaci is endemic to the highland forests of Kenya. The species’ global population declined in the 20th century because of habitat loss, hunting pressure and disease (Kingdon, Reference Kingdon1982). Although there are no reliable abundance estimates, it is believed that < 100 mountain bongos remain in the wild, and thus the subspecies is categorized as Critically Endangered on the IUCN Red List (IUCN SSC Antelope Specialist Group, 2017b).
Bongos Tragelaphus eurycerus are habitat specialists, with a clear preference for forest and secondary growth (Kingdon, Reference Kingdon1982; Estes et al., Reference Estes, Okin, Mwangi and Shugart2008; Elkan & Smith, Reference Elkan, Smith, Kingdon and Hoffmann2013), and the nominal subspecies Tragelaphus eurycerus eurycerus, the lowland or western bongo (found in West and Central Africa), is found throughout the equatorial forest. It is more common than the mountain bongo and is categorized as Near Threatened on the IUCN Red List (IUCN SSC Antelope Specialist Group, 2017a). The mountain bongo is considered a relic of the Pleistocene forest cover fluctuations in East Africa (Moreau, Reference Moreau1933), a phenomenon common amongst other typically Central African species (Kingdon, Reference Kingdon1982). It was once common across forests in the East African highlands from Mount Elgon (on the Kenyan border with Uganda) to the Cherangani Hills (Price, Reference Price1969; Kingdon, Reference Kingdon1982), but its current range is limited to four mountain areas, all in Kenya: Maasai Mau, Eburu, the Aberdares and Mount Kenya (Faria et al., Reference Faria, Kavembe, Jung'a, Kimwele, Estes and Reillo2011). Moreover, the forests of central Kenya are limited to the highlands, as lower elevations have been converted to extensive agriculture, rendering the few areas still occupied by the mountain bongo (hereinafter bongo) completely disconnected.
There is a lack of information regarding remnant bongo populations in Kenya, in part because the subspecies inhabits difficult terrain in montane forests and exhibits elusive behaviour, which together make sightings rare. Hence, although bongos have long been a focus of international conservation efforts, information on the status of populations in the wild is sparse. To date, monitoring of the remnant subpopulations has relied on surveillance monitoring. A local NGO, the Bongo Surveillance Project, has conducted camera trapping to confirm presence at known locations of occurrence since the early 2000s (Prettejohn, Reference Prettejohn2004, Reference Prettejohn2008; Plate 1). Although this surveillance has been essential in confirming continued bongo presence, more detailed information is needed to manage these subpopulations effectively.

Plate 1 The mountain bongo Tragelaphus eurycerus isaaci recorded by a camera trap at a salt lick in the Salient area of Aberdare National Park, Kenya. The Bongo Surveillance Project places cameras at active salt licks to maximize encounter probability.
Combining camera trapping with mark–recapture (in which individuals are either physically marked or are identifiable noninvasively through, for example, unique natural markings; Petit & Valiere, Reference Petit and Valiere2006) allows estimation of vital parameters such as survivorship, recruitment and population growth rate (Lebreton et al., Reference Lebreton, Pradel and Clobert1993; Pradel, Reference Pradel1996). The bongo is characterized by markings on its flanks, chest and limbs (Elkan & Smith, Reference Elkan, Smith, Kingdon and Hoffmann2013), and there is evidence that these markings are informative for individual identification (Gibbon et al., Reference Gibbon, Bindemann and Roberts2015).
Our overall aim in this study was to retrieve estimates of bongo population size from historical camera-trap data and thus evaluate the status of remnant subpopulations to inform conservation management (Yoccoz et al., Reference Yoccoz, Nichols and Boulinier2001). Because monitoring by the Bongo Surveillance Project has taken place over several years, population size can be estimated for each year, thus facilitating the estimation of population trends.
Our specific objectives were to (1) identify individuals in camera-trap records collected by the Bongo Surveillance Project, and (2) conduct mark–recapture analysis to provide estimates of the population size, trends and vital parameters of the remnant bongo subpopulations. We discuss our results in the context of ongoing conservation management efforts for this species (KWS, 2019).
Methods
Historical camera-trap data and identification scoring
We analysed camera-trap images collected by the Bongo Surveillance Project during 2013–2018. The areas surveyed consist of the only four areas where bongos have ever been encountered both through genetic surveys (Faria et al., Reference Faria, Kavembe, Jung'a, Kimwele, Estes and Reillo2011) and through camera-trap surveys. The Bongo Surveillance Project has conducted additional surveys in other areas (north and south Aberdares and other parts of the Mau Forest complex and Mount Kenya), but these failed to find bongos. The areas where bongos were located during the period of interest (2013–2018) and the areas included here are: Salient section of the Aberdares (c. 70 km2 within the 767 km2 of Aberdare National Park and the contiguous forest reserve), Eburu (c. 60 km2), Maasai Mau (a section of c. 300 km2 of the wider Mau Forest complex) and Mount Kenya in the proximity of Ragati Conservancy (within Mount Kenya National Park and the contiguous forest reserve, c. 1,420 km2). Figure 1 shows the locations of these monitoring sites. We focused on those surveys where bongo presence had been confirmed previously, and the camera-trap record results from 78 discrete surveys conducted in the four areas known to host bongos: Aberdares (24 surveys), Mount Kenya (22), Maasai Mau (19) and Eburu (13). Each discrete survey lasted 3–4 weeks, with 1–3 cameras placed at salt licks known through indirect signs such as dung and spoor to be frequented by bongos. The Bongo Surveillance Project monitors two salt licks in the Aberdares and one in each of the other areas using Bushnell Natureview HD and Essential HD cameras (Bushnell, Overland Park, USA). Cameras were set to take three photos per capture and were active for the whole 3–4 week period. An infrared flash was used for night-time captures.

Fig. 1 The four isolated mountain areas monitored by the Bongo Surveillance Project in central Kenya. These are the only areas where the Project has recorded the mountain bongo Tragelaphus eurycerus isaaci.
We used our visual identification system (Sandri et al., Reference Sandri, Bunge, Omengo, Jones, Cain and Harris2023) to identify individual flanks. Bongos are asymmetrical in their coat pattern, so we could not match the left and right flanks of individual animals (the historical camera-trap data we had access to were captured using a single-camera design). Hence, the flank rather than the individual was the subject of our capture histories, where the first capture counts as marking and further detections as recaptures. We split the data into two capture histories for each area: one for left flanks and one for right flanks. We analyse these capture histories separately (Wang & Macdonald, Reference Wang and Macdonald2009). We excluded flanks encountered just once from the analysis as they could result from misidentification and so bias survival estimates (Morrison et al., Reference Morrison, Yoshizaki, Nichols and Bolger2011).
Bongos are characterized by sexual dimorphism in both size and appearance. Females weigh 250–300 kg and males can exceed 400 kg. Both sexes have horns, but these grow larger and are more divergent in males, and males are also characterized by a darker coat colouration when mature (Elkan & Smith, Reference Elkan, Smith, Kingdon and Hoffmann2013). We estimated bongo age, recorded as the age at first capture (i.e. age at marking), according to horn development, a method also used with other antelopes (Owen-Smith, Reference Owen-Smith1993; Marshal, Reference Marshal2017). We implemented two age classes (immature: < 2 years; adult: ≥ 2 years) following methods described previously (Pollock, Reference Pollock1981). We sexed adult flanks according to horn shape and coat colour (Elkan & Smith, Reference Elkan, Smith, Kingdon and Hoffmann2013; Castelló, Reference Castelló2016), and we sexed immature individuals by observing the orientation of growing horns (divergent in males, almost parallel in females).
Population analysis
We analysed capture histories using the Robust Design model in MARK (Pollock, Reference Pollock1982; Huggins, Reference Huggins1989; White & Burnham, Reference White and Burnham1999; Kendall, Reference Kendall, Cooch and White2018) to estimate probabilities of survival, first detection and recapture, temporary emigration, and population size as a derived parameter. As the four areas surveyed are not connected and the discrete surveys involved single cameras in single locations, we lacked the conditions to include a spatial component in the analysis. We considered each year during 2013–2018 as a primary occasion and each discrete survey as a single secondary occasion. We tested the Robust Design assumption of population closure for each of the primary periods included in the analysis, using the Stanley–Burnham test (Stanley & Burnham, Reference Stanley and Burnham1999) in CloseTest (Stanley & Richards, Reference Stanley and Richards2005). We did not include in this analysis any annual population estimates that violated the closure assumption. We also excluded three surveys from 2018 that failed to meet the assumption of an open population between the latest primary occasions (2017 and 2018). The surveys included in the analysis are shown in Table 1.
Table 1 Years and numbers of surveys of the mountain bongo Tragelaphus eurycerus isaaci conducted by the Bongo Surveillance Project in Kenya (Fig. 1), with the interval (in months) between each survey and the previous one.

To compensate for the lack of detailed life history information for the bongo, we evaluated competing models in MARK, allowing all parameters to be dependent on time (i.e. primary occasion), sex and age at marking. The influence of age at marking was assessed by applying age models (Pollock, Reference Pollock1981). We incorporated current knowledge of bongo sociality and ecology: females are highly social and move in herds with their young, whereas mature males are solitary and only occasionally join herds of females for breeding purposes, without, however, coercing or limiting their movement (Estes, Reference Estes1992; Elkan & Smith, Reference Elkan, Smith, Kingdon and Hoffmann2013). Therefore, we modelled capture and migration parameters as being equal in adult females and immature individuals, as these are known to move together (Kingdon, Reference Kingdon1982; Estes, Reference Estes1992). Following Kendall (Reference Kendall, Cooch and White2018), prior to conducting the analysis we assessed the goodness of fit of a baseline model containing all parameters for the capture histories of both areas. We used the bootstrap GOF function in MARK with 1,000 iterations, after which we calculated the probability of a model with a higher deviance than the original as a measure of model fit. We followed an information-theoretic approach to conduct model selection by relying on the small-sample corrected Akaike information criterion (AICc), and we deemed informative only models within ΔAICc ≤ 4 of the best model (Anderson & Burnham, Reference Anderson and Burnham2002). Finally, we used model averaging across the parameters for the subset of best models to estimate vital rates and population size. Implementing model averaging can lead to confused or biased conclusions when many predictors are used (Cade, Reference Cade2015). Therefore, care must be taken in interpreting model averaging outputs (Dormann et al., Reference Dormann, Calabrese, Guillera-Arroita, Matechou, Bahn and Bartoń2018), and our use of a limited set of ecologically based predictors helps with such interpretation.
We assessed the trends of the surveyed populations during 2013–2018 by calculating yearly population growth rate (λ), calculated as ![]() $ \hat{ N}( t) / \hat{ N}( t-1) $, where
$ \hat{ N}( t) / \hat{ N}( t-1) $, where ![]() $ \hat{ N} $ is the population size estimate and t is the primary period (Owen-Smith & Mason, Reference Owen-Smith and Mason2005). Population parameter estimates were similar for each flank; we assessed population growth by using the estimate of either the right or left flank depending on which presented the lowest standard error of the estimate.
$ \hat{ N} $ is the population size estimate and t is the primary period (Owen-Smith & Mason, Reference Owen-Smith and Mason2005). Population parameter estimates were similar for each flank; we assessed population growth by using the estimate of either the right or left flank depending on which presented the lowest standard error of the estimate.
Results
We identified 102 unique flanks in the footage spanning 2013–2018 in the four areas of interest. Of these, we encountered eight once only, and thus we excluded these from the mark–recapture analysis. We identified a single female flank in Mount Kenya and a single male flank in Eburu, and thus no capture history was possible for these areas. Hence, we conducted a mark–recapture analysis only for the Aberdares and Maasai Mau populations (Table 2).
Table 2 Number (left:right flanks), number of female and male (number of flanks recorded as immature), abundance estimates (![]() $ \hat{ N} $) and sex ratios of mountain bongo flanks in each year in the two areas included in the mark–recapture analysis (Aberdares and Maasai Mau) in Kenya. The sex ratio refers to adult individuals. Population growth (λ) from one period to the next is also shown; values in parentheses refer to λ calculated from lower and upper limits of the 95% CIs of the abundance estimate (
$ \hat{ N} $) and sex ratios of mountain bongo flanks in each year in the two areas included in the mark–recapture analysis (Aberdares and Maasai Mau) in Kenya. The sex ratio refers to adult individuals. Population growth (λ) from one period to the next is also shown; values in parentheses refer to λ calculated from lower and upper limits of the 95% CIs of the abundance estimate (![]() $ \hat{N} $). No population growth values are given for periods 1 and 2 in Maasai Mau because there were insufficient numbers of capture occasions (see text for details).
$ \hat{N} $). No population growth values are given for periods 1 and 2 in Maasai Mau because there were insufficient numbers of capture occasions (see text for details).

Per mark–recapture analysis, our most informative models (within ΔAICc ≤ 4) are shown in Table 3. Here we assume survivorship, temporary emigration and capture probability are dependent on both sex and age. Although we implemented model averaging in retrieving estimates, we consider it of interest to compare the influence of sex and age on the parameters. Capture probability is shaped by sex and age in all models, thus reflecting the behavioural ecology of bongos, with females and young living in herds whereas adult males are solitary. This is confirmed when assessing temporary emigration, which we found to be influenced by sex and the age of males in three of the five best models. Survivorship is also influenced by both sex and age in all models. However, age seems to have a more powerful influence as the model not including age in shaping survivorship is the least informative, with a ΔAICc of 4.07 (Table 4). We modelled all parameters as constant across the period of interest (2013–2018). Estimates for each area, age and sex class are presented in Table 5.
Table 3 The most informative (within ΔAICc ≤ 4 of the most informative model) of the 20 a priori models run in MARK, which include sex and age as critical in shaping survival of mountain bongos in Kenya.
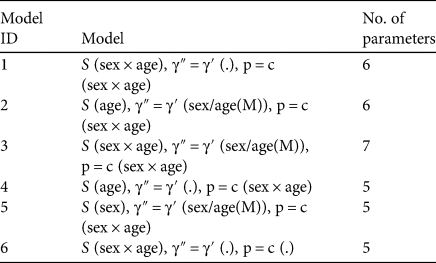
S, apparent survival.
γ″ = γ′, random movement.
(.), model with no sex or age dependence.
(sex), the parameter is modelled as sex dependent.
(age), the parameter is modelled as age dependent.
(sex × age), the parameter is modelled as dependent on both sex and age.
(sex/age(M)), the parameter is dependent on sex for all groups but only on age for males.
p = c, capture probability equals recapture probability.
Table 4 The most informative models of mountain bongo survival in Kenya (described in Table 3) ranked according to their AICc for each of the three datasets (Aberdares left and right flanks; Maasai Mau left flanks). Although the ranking of the models is different between the datasets, the best models always include age and sex as relevant factors in shaping survival in both areas.
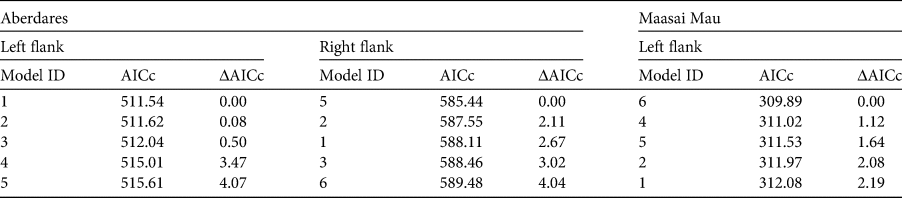
Table 5 Estimates for survival (S), temporary emigration (γ″) and capture probability (p) of mountain bongos in Kenya averaged amongst the most informative models (within ΔAICc ≤ 4 of the best overall model) for every age and sex class. Estimates of temporary emigration for immature individuals are modelled as being equal to those of adult females because immature individuals reside in the herd regardless of their sex.

Population size estimates for the Aberdares and Maasai Mau are presented in Table 2. The largest population was in the Aberdares, with an estimate of 23.11 (95% CI 17.11–29.10) individuals, whereas 16.47 (95% CI 12.16–20.78) individuals were estimated in Maasai Mau. When estimating population size, we found the capture history of left flanks from the first survey period in the Aberdares to violate the assumption of closure (Stanley–Burnham test, P < 0.05) and therefore we ignored this capture history. Capture histories from Maasai Mau for primary occasions 1 and 2 both comprise only two capture occasions, which is insufficient for the closure test and hence we ignored estimates from these primary occasions. Population size estimates for each primary occasion and the sex ratio of individuals encountered in the Aberdares and Maasai Mau are shown in Table 2. We calculated population growth as yearly λ (Table 2). Both populations have λ estimates of > 1.0 (i.e. positive population growth rate) in the period of interest (2013–2018; Fig. 2), although estimates for Maasai Mau are limited to the latest primary occasions (2016–2018; Fig. 2).

Fig. 2 Estimated numbers of the mountain bongo in Aberdares and Maasai Mau from 2013 to 2018. Although the population trends suggest a growing population, the large 95% CIs and the limited time period in Maasai Mau do not allow us to confidently assess the population trends.
Discussion
Here we present the first direct evaluation of population status and growth trends for the mountain bongo in the wild through the combination of historical camera-trap data with our novel, repeatable individual identification system and mark–recapture analysis. We report the estimated population sizes of Maasai Mau and the Aberdares populations for the first time (Table 2). Our results indicate that the combined bongo population in the Aberdares and Maasai Mau is likely to be 29–50 individuals in total (based on the lower and upper limits of the confidence intervals for the two areas combined). Kingdon (Reference Kingdon1982) reported herds of > 50 in the Aberdares in the 1980s, and this suggests the current situation is not ideal for this antelope. Moreover, implementing mark–recapture allowed us to recognize the influence of sex and age on survivorship in bongos (Table 5). The information presented here could help with conservation planning and management decisions.
Our total population size estimate is lower than that reported by IUCN (2017b), highlighting the critical situation facing this antelope in the wild. Although the largest population of the Aberdares resides entirely within a national park and therefore could be assumed to be fully protected, Maasai Mau is not fully protected by a national park or reserve, and evidence of its importance for this endemic Kenyan species could help those advocating for the conservation of the entire Mau Forest complex (Nkako et al., Reference Nkako, Lambrechts, Gachanja and Woodley2005). These estimates will provide valuable information for managers of these wild populations, as knowledge about the species in the wild is a priority outlined in the bongo recovery and action plan (KWS, 2019). Moreover, although we did not reconstruct population networks within the two areas, it appears that in both Maasai Mau and the Aberdares all individuals belonged to a single herd, as the same individuals were systematically encountered together.
When working with small populations of rare or elusive species, relying on sites of known activity is vital, and the Bongo Surveillance Project approach of camera trapping at salt licks is thus strategic. Salt licks are known to be relevant for bongo ecology (Klaus et al., Reference Klaus, Klaus-Hügi and Schmid1998; Klaus-Hügi et al., Reference Klaus-Hügi, Klaus, Schmid and König1999, Reference Klaus-Hügi, Klaus and Schmid2000), and the approach of relying on these locations to study bongo populations has been used previously (Hillman, Reference Hillman1986). The same approach has also been used successfully to study species other than ungulates (e.g. the spider monkey Ateles belzebuth; Galvis et al., Reference Galvis, Link and di Fiore2014), and its accuracy was comparable to that of direct sightings along transects. A limitation of the approach followed here is the impossibility of including a spatial component in the analysis (Royle et al., Reference Royle, Chandler, Sollmann and Gardner2014), which would help with defining the home ranges of herds and individuals. However, given the small size of the population, the inclusion of a spatial component (i.e. an array of camera traps) would be prohibitive in both economic and logistical terms because of the difficult terrain and the large number of traps needed. A possible solution would be to monitor an array of licks, which would help with refining the area used by a particular herd, as it appears that bongos rely on multiple licks (Klaus-Hügi et al., Reference Klaus-Hügi, Klaus and Schmid2000).
We assessed population structure in both the Aberdares and Maasai Mau through sex ratios (Table 2). A skewed ratio in favour of females is typical of antelopes (Jarman, Reference Jarman1974). The sex ratio in the Aberdares population is similar to that of other African antelopes for which males are mostly solitary (Owen-Smith & Mason, Reference Owen-Smith and Mason2005). However, considering the small size of these populations, a skewed sex ratio could be problematic, as a low number of males could lead to females failing to conceive in a given season, with a consequent effect on population dynamics (Milner-Gulland et al., Reference Milner-Gulland, Bukreeva, Coulson, Lushchekina, Kholodova, Bekenovil and Grachev2003; Rankin & Kokko, Reference Rankin and Kokko2007).
Our finding that the probability of emigration is higher in males than in females, combined with their lower encounter probability, could indicate that males are more likely to leave the pool of individuals that could potentially be encountered. Males might not visit salt licks as often as females and this could result in the lower encounter probability, which has also been observed in previous research (Hillman, Reference Hillman1986). Nevertheless, the lack of encounters of solitary males during surveys in other areas of the Aberdares (M. Prettejohn, pers. comm., 2018; Sandri, Reference Sandri2020) suggests that further analysis using alternative methods or more extensive camera trapping is needed to gain a clearer picture of male movements. Moreover, although the Bongo Surveillance Project has conducted multiple surveys in most of the historical bongo range, additional surveys should be a priority, to assess whether other as yet undiscovered populations still reside in Kenya and beyond. Considering this, we define ours as an estimate of the known bongo population, but we do not infer presence or extrapolate abundance to other areas of the former bongo range because of the low likelihood of locating other populations of the bongo.
Although our sample size was limited, the lack of large predators could explain the overall high survival rates (> 0.8) of both sexes, as these appear closer to the rates found in ungulates that inhabit temperate areas with no large predators (Gaillard & Yoccoz, Reference Gaillard and Yoccoz2003) than those typical of large herbivores in African savannahs (Owen-Smith & Mason, Reference Owen-Smith and Mason2005). However, these high estimates of survival refer to individuals encountered at surveyed salt licks (0.79–0.99; Table 5). This supports the suggestion that the gregariousness of females might protect calves from predators. Nevertheless, calves encountered at licks might not represent all the calves of the year because bongos keep their young separated from the herd for at least 2 weeks following birth (Kingdon, Reference Kingdon1982). Mortality during this crucial period could not be estimated in our study, and this might have a larger influence on population dynamics than the survival of older calves once they join maternal herds and start visiting licks (Hillman, Reference Hillman1986).
The work presented here provides future conservation actions, such as the reintroduction of captive-bred individuals, with a framework for monitoring newly established populations with minimal disturbance by using camera traps placed at salt licks paired with a visual identification system. This could be enhanced by using marking techniques (ear notches or tags), which would further reduce misidentification. Moreover, our work shows that the use of regularly visited sites can help conservationists and managers to monitor otherwise difficult-to-encounter wild forest ungulates for which vital rate estimates would be valuable.
Our results contribute new information to our knowledge of the critical situation facing bongos in the wild, with > 40 individuals remaining in two isolated populations. The limited evidence of other populations in the remaining range (i.e. Mount Kenya and Eburu), where the surveys failed to encounter bongo herds, may be a result of low survey effort. Because of the limited sample size and high 95% CIs of the estimates (particularly for Maasai Mau; Fig. 2), we are unable to draw conclusions regarding any trends in these populations. Nevertheless, our findings highlight the relevance of the Masai Mau forest for the long-term conservation of this antelope, which is a flagship species for the entire Afro-montane ecosystem. The identification system (Sandri et al., Reference Sandri, Bunge, Omengo, Jones, Cain and Harris2023) has been included as an appendix to the Kenyan national strategy for the bongo (Sandri et al., Reference Sandri, Omengo, Cain, Jones, Mallon and Harris2019), and documentation of the identified individuals has been shared with both Kenya Wildlife Service and the Bongo Surveillance Project, to facilitate the continued monitoring of the remnant bongo populations. However, more recent surveys in Eburu and Mount Kenya have failed to provide evidence of bongo presence, whereas the herds encountered in the Aberdares and Maasai Mau are still being encountered (M. Prettejohn, pers. comm., 2018).
Although we emphasize the need for further monitoring of these remnant populations, in light of the critical situation of the bongo we are aware of the risk of ‘counting the books while the library burns’ (Lindenmayer et al., Reference Lindenmayer, Piggott and Wintle2013). Considering the successful captive breeding programme conducted by zoos, with > 700 individuals (Bosley, Reference Bosley2016), our analysis needs to be used to elicit conservation actions aimed at reinforcing or establishing additional populations across the species’ range.
Author contributions
Conceptualization, design: TS, BC, WEH; data collection: MP; analysis and interpretation: TS; writing, revision: all authors.
Acknowledgements
We thank the Bongo Surveillance Project team for their help and support in making their data available for this analysis. The identification system development benefitted from the input of MSc student David Hool. We thank two anonymous reviewers for their comments and suggestions. This work was made possible through the support of Manchester Metropolitan University and Chester Zoo.
Conflicts of interest
None.
Ethical standards
The research was supported by a scholarship from Manchester Metropolitan University, Manchester, UK. The university ethics committee did not recognize any ethical issues. An agreement on data sharing was signed between Manchester Metropolitan University and the Bongo Surveillance Project. This research otherwise abided by the Oryx guidelines on ethical standards.
Data availability
The data used for this analysis are not publicly available.












