Introduction
Sepsis is the result of a complex interaction between the host response and infecting organism resulting in life-threatening organ dysfunction( Reference Singer, Deutschman and Seymour 1 ). The host response results in a systemic inflammatory syndrome that manifests as hyper/hypothermia, tachycardia, tachypnea and alterations in the leucocyte count( Reference Bone, Balk and Cerra 2 ). When this response becomes pathological, it leads to microcirculatory derangements( Reference Trzeciak, Dellinger and Parrillo 3 ), hypotension, cellular hypoxia/dysoxia( Reference Loiacono and Shapiro 4 ) and ultimately death. It is estimated that 1·7 million adults were hospitalised in the USA in 2014 with sepsis( Reference Rhee, Dantes and Epstein 5 ), which results in a major economic burden given that it is the most expensive condition to treat in US hospitals( Reference Torio and Moore 6 ). Sepsis is the leading cause of mortality in hospitalised patients, contributing to 1 in every 2–3 deaths( Reference Liu, Escobar and Greene 7 ). Despite its invasive nature and high mortality rates, every targeted, large pharmaceutical trial has failed( Reference Marshall 8 ).
Sepsis results in a high energy expenditure due to its elevated metabolic demand as well as inefficiencies in normal biochemical processes( Reference L’Her and Sebert 9 ). The hypermetabolic state induced by the host response to the infection as well as the need to replenish damaged cells contributes to micronutrient deficiencies. The resulting deficiencies lead to alterations in normal energy homeostasis and inefficiencies in energy production. The standard of care to treat sepsis is early intravenous fluids, prompt broad-spectrum antibiotics, source control of the infecting agent, low tidal volume ventilation and haemodynamic optimisation to maintain an adequate blood pressure to perfuse end-organs( Reference Rhodes, Evans and Alhazzani 10 ). However, a successful adjunctive treatment to curtail the inflammatory response, diminish free-radical damage and/or improve metabolic derangements would result in a much-needed weapon in the fight against sepsis. Micronutrient therapy has the potential to aid in such processes making it an interesting subject to study. We present the current state of knowledge on micronutrients in sepsis as it pertains to thiamine, l-carnitine, vitamin C, Se and vitamin D, as these have been extensively studied in the field of sepsis (Table 1).
Table 1 Summary table of micronutrient literature in sepsis
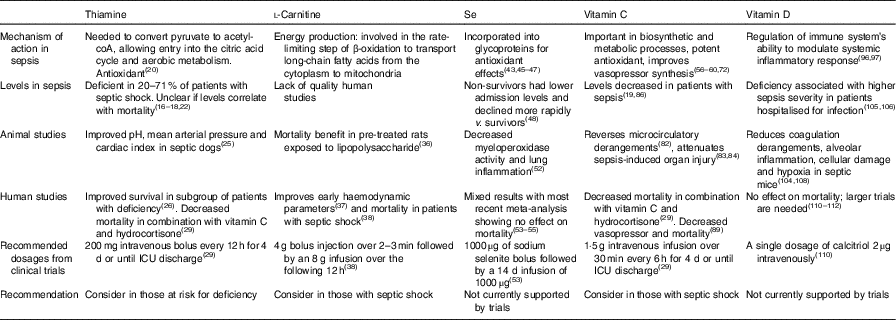
ICU, intensive care unit.
Thiamine
Thiamine serves as a cofactor for multiple cellular enzymes that are essential in aerobic carbohydrate metabolism, maintenance of cellular redox status, mitochondrial oxidative phosphorylation and synthesis of adenosine triphosphate( Reference Manzanares and Hardy 11 ). Despite its importance, the body does not produce thiamine and can only store up to 30 mg at a time in tissues such as the skeletal muscle, heart, kidney and brain( Reference Ariaey-Nejad, Balaghi and Baker 12 ). Due to its quick turnover, thiamine deficiency can develop within 2 weeks without adequate supplementation. Many well-described syndromes are known to result from deficiencies of thiamine, including cardiac beriberi and Wernicke’s encephalopathy( Reference DiNicolantonio, Niazi and Lavie 13 , Reference Krill 14 ). Thiamine is found in raw foods including green vegetables and nuts as well as certain processed foods such as fortified cereals( Reference Depient, Bruce and Shangari 15 ).
Thiamine deficiency is commonly found among septic patients, with a range in prevalence between 20 and 71 %, depending on measurement techniques and inclusion criteria( Reference Donnino, Carney and Cocchi 16 – Reference Dizdar, Baspinar and Kocer 18 ). Donnino et al. ( Reference Donnino, Carney and Cocchi 16 ) found that 20 % of septic patients exhibited thiamine deficiency, where sepsis was defined as suspected infection and evidence of tissue hypoperfusion (lactate>4 mmol/l or hypotension requiring vasopressor support); an absolute thiamine deficiency was noted at a level<9 nmol/l. In another study, Costa et al. ( Reference Costa, Gut and de Souza Dorna 17 ) showed that the incidence of thiamine deficiency was 71·3 % in septic shock patients, defined as hypotension requiring vasopressor support. A serum thiamine below 16 ng/ml was set as the threshold below which thiamine deficiency was defined. Lastly, Dizdar et al. ( Reference Dizdar, Baspinar and Kocer 18 ) found that 56 % of septic patients exhibited thiamine deficiency, with an average serum concentration of 28·3 ng/ml (normal range: 33–99 ng/ml). In this work, sepsis was again defined as an infection-induced inflammatory response resulting in hypotension requiring vasopressor support. Although these studies were mostly small, uni-centre undertakings, they demonstrate that, despite large variability, thiamine deficiency is prevalent in the sepsis population.
There are multiple possible mechanisms to explain the association between thiamine deficiency and sepsis, though whether the deficiency is a cause or consequence of sepsis is not evident. Decreased appetite, diarrhoea, impaired absorption, and/or increased metabolic demand could deplete thiamine reserves and contribute to thiamine deficiency during sepsis. By decreasing the activity of the pyruvate dehydrogenase complex (needed to convert pyruvate to acetyl-coA in order to enter the citric acid cycle), thiamine deficiency can increase lactic acid production by favouring the anaerobic metabolism of pyruvate and shunting it away from the citric acid cycle (Fig. 1). Further, the profound oxidative stress that is a hallmark of sepsis can result in consumption of endogenous antioxidants( Reference Goode, Cowley and Walker 19 ). Thiamine, with its role in preventing lipid peroxidation and oleic acid oxidation, is one such antioxidant( Reference Lukienko, Mel’nichenko and Zverinskii 20 ). Animal studies may also provide clues regarding this association. In an experimental model of sepsis, thiamine deficiency was associated with oxidative stress and inflammatory changes( Reference De Andrade, Gayer and Nogueira 21 ). In this paper, mice in which sepsis was induced via caecal ligation and puncture were fed a diet deficient in thiamine; these mice displayed increases in inflammatory and oxidative stress markers compared with controls. This suggests that thiamine deficiency can compound the body’s inherent stress response to sepsis.
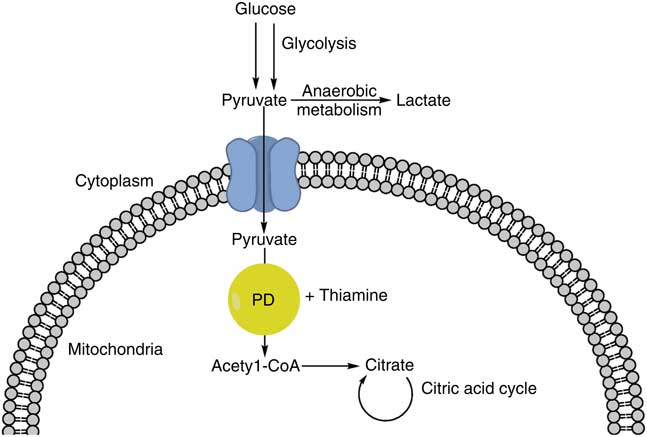
Fig. 1 Glucose undergoes glycolysis, forming pyruvate. Pyruvate can then be converted to acetyl-CoA via pyruvate dehydrogenase (PD) and thiamine, entering the citric acid cycle. Alternatively, pyruvate can undergo anaerobic metabolism and form lactic acid.
Despite the prevalence of thiamine deficiency in sepsis, studies have found conflicting relationships between thiamine levels and clinical outcomes. One study found that those with lower levels of thiamine had an increased mortality rate( Reference Cruickshank, Telfer and Shenkin 22 ) while others failed to find any such link( Reference Dizdar, Baspinar and Kocer 18 ). A potential drawback of the first study was that it was retrospective, conducted only in intensive care unit (ICU) patients requiring parenteral nutrition support, and used an indirect measurement of thiamine activity. In a prospective observational study of 125 patients admitted to the ICU, Corcoran et al. ( Reference Corcoran, O’Neill and Webb 23 ) examined the association of vitamin deficiency with mortality and found no significant association. A potential drawback of this study was that only nine of the 125 patients had a measurable thiamine deficiency. Similarly, Dizdar et al. ( Reference Dizdar, Baspinar and Kocer 18 ) found that while thiamine levels were reduced in patients who died, the levels were not independently associated with mortality. Therefore, more studies with larger sample sizes are needed to draw a definite conclusion.
Due to the prevalence of thiamine deficiency and its possible effect on clinical outcomes in septic patients, studies have been undertaken to determine the therapeutic relevance of thiamine administration. Using cultured rat cardiomyocytes, Shin et al. ( Reference Shin, Choi and Cho 24 ) demonstrated that thiamine can be cytoprotective in cells undergoing hypoxic stress by preventing apoptosis. An early animal study corroborated the idea that thiamine administration was beneficial in septic shock. Thiamine pyrophosphate administration improved pH, mean arterial pressure and cardiac index in a dog model of endotoxic shock( Reference Lindenbaum, Larrieu and Carroll 25 ). More recent clinical research has shown mixed results. In the largest human randomised, double-blind clinical study investigating the therapeutic potential of thiamine to date, Donnino et al. ( Reference Donnino, Andersen and Chase 26 ) found that administration of thiamine did not improve lactate levels, mortality or ICU length of stay in patients with septic shock and elevated lactate. In this study, a plasma thiamine level ≤ 7 nmol/l was considered deficient and a dose of 200 mg of thiamine intravenously was administered twice daily for 7 d or until hospital discharge. However, when baseline thiamine deficiency was taken into account, patients had a significantly lower lactate level at 24 h and longer survival rates. A secondary analysis of the study indicated that those randomised to the thiamine group had lower creatinine levels and a lower rate of progression to renal replacement therapy( Reference Moskowitz, Andersen and Cocchi 27 ). This study suffers from some limitations, including the fact that the subgroup of thiamine-deficient patients was very small (fifteen in treatment, thirteen in control) and was no longer randomised. In support of Donnino’s findings that thiamine-deficient septic patients could benefit from thiamine supplementation was a retrospective study in which patients with alcohol use disorder (known to be associated with lower levels of thiamine) who were given thiamine during their hospital stay had a decreased mortality rate from septic shock( Reference Holmberg, Moskowitz and Patel 28 ). Additionally, Marik et al. ( Reference Marik, Khangoora and Rivera 29 ) showed that the combination of hydrocortisone (50 mg every 6 h for 4 d or until ICU discharge), vitamin C (1·5 mg every 6 h for 4 d or until ICU discharge) and thiamine (200 mg every 12 h for 4 d or ICU discharge) had a significant effect on patients with sepsis and septic shock. Patients receiving the intervention had a marked reduction in mortality (8·5 % (four out of forty-seven) compared with 40 % (nineteen out of forty-seven) of controls), where all four of the patients in the treatment group were deemed to have died from non-sepsis-related causes. Further, there was a significant decrease in time to weaning of vasopressor support in the treatment group compared with controls. The authors purport that thiamine was added since it is a co-enzyme for vitamin C metabolism and that the encouraging results can be attributed to the synergistic effects of hydrocortisone and vitamin C.
The above studies together indicate that the administration of thiamine, probably in conjunction with other micronutrients, may be beneficial in the septic patient with risk factors for thiamine deficiency, as intravenous thiamine is cheap and has relatively few side effects. Such risk factors for thiamine deficiency include malnutrition, alcoholism, chronic wasting diseases, renal replacement therapy, hyperemesis gravidarum, anorexia nervosa, gastric bypass surgery and refeeding( Reference Leite and de Lima 30 ). Due to the small sample sizes and drawbacks of the aforementioned studies, we instead feel that the clinician may consider thiamine supplementation in the septic shock patient with risk factors for thiamine deficiency. Future studies may benefit from elucidating whether altered thiamine utilisation and uptake by various tissues during sepsis may be a critical determinant of whether patients benefit from thiamine administration.
l-Carnitine
Fatty acids and TAG are utilised as energy in a process known as β-oxidation. The rate-limiting step in this process requires an l-carnitine-mediated transport of long-chain fatty acids from the cytoplasm into the mitochondria (Fig. 2). Carnitine palmitoyl transferase 1 is the enzyme responsible for this transport and its activity is inhibited during sepsis( Reference Eaton, Fukumoto and Stefanutti 31 ).
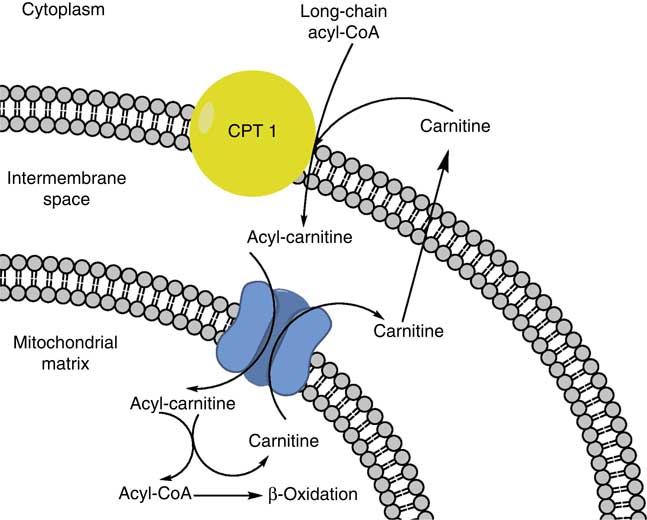
Fig. 2 Long-chain acyl-CoA is shuttled into the mitochondrial matrix for β-oxidation by combining with carnitine via carnitine palmitoyl transferase 1 (CPT 1).
l-Carnitine is primarily obtained from food, with meat being the largest source; however, it can be endogenously synthesised from the amino acids lysine and methionine( Reference Tanphaichitr and Broquist 32 , Reference Tanphaichitr, Horne and Broquist 33 ). Systemic primary carnitine deficiency is characterised by episodes of metabolic crisis characterised by hypoketotic hypoglycaemia events, hyperammonaemia, hypertransaminasaemia, hepatomegaly and hepatic encephalopathy. In addition, skeletal muscle weakness, cardiomyopathy and elevated creatinine kinase levels can be seen( Reference Longo, di San Filippo and Pasquali 34 , Reference Roe and Ding 35 ).
The first animal study investigating the link between l-carnitine and sepsis was published in 1989 and showed a mortality benefit in pre-treated l-carnitine rats exposed to a lipopolysaccharide (LPS) sepsis model( Reference Takeyama, Takagi and Matsuo 36 ). Two human clinical trials have evaluated the use of l-carnitine in sepsis and both showed promising results. The first, published in 1991, demonstrated early improvements in haemodynamic parameters in patients with septic shock( Reference Gasparetto, Corbucci and de Blasi 37 ). The second, a small phase 1 randomised control trial of thirty-one patients, analysed the safety and efficacy of l-carnitine infusion in patients undergoing vasopressor-dependent septic shock. This study showed a lower 28 d mortality rate of 25 v. 60 % favouring l-carnitine with no differences in significant adverse events( Reference Puskarich, Kline and Krabill 38 ). The dosage for l-carnitine in the later study was a 4 g bolus injection (20 ml) over 2–3 min followed by an 8 g infusion (8 g in 1000 ml of 0·9 % normal saline) over the following 12 h (83 ml/h). A phase 2 trial with an estimated 250-patient enrollment is currently underway( Reference Lewis, Viele and Broglio 39 ).
In conclusion, preliminary results merit consideration for further research in this area and clinicians may consider l-carnitine supplementation for patients in septic shock.
Selenium
Se is an essential nutrient required for life( Reference Bösl, Takaku and Oshima 40 ). The recommended daily allowance is 55 μg/d, with the median American ingesting 81 μg/d. It is widely available and found in dairy products, meats, seafood, vegetables and grains( 41 ), with regional differences in the soil resulting in variations in consumption across geographical areas( Reference Thompson, Erdody and Smith 42 ). The majority of Se is incorporated into glycoproteins( Reference Burk and Hill 43 ), with the liver being the major source of secretion( Reference Hill, Zhou and McMahan 44 ). For example, the glycoprotein glutathione peroxidase requires Se at its active site to detoxify reactive oxygen species such as H2O2 and phospholipid hydroperoxide( Reference Epp, Ladenstein and Wendel 45 , Reference Lei, Cheng and McClung 46 ). Se is frequently referred to as an antioxidant due to its role in reversing the effects of oxidised lipids and methionine residues, and detoxifying hydrogen peroxidase( Reference Huang, Rose and Hoffmann 47 ).
Sakr et al. ( Reference Sakr, Reinhart and Bloos 48 ) studied the time course of Se levels in sixty surgical ICU patients with four equal subgroups consisting of ICU-controls, systemic inflammatory response syndrome (SIRS), severe sepsis, and septic shock ICU patients. He found that 92 % of patients had Se levels below the standard for healthy subjects (74 μg/l) and that all but the ICU-control cohort had declining levels of Se during their ICU stay. Se levels were lowest on admission and decreased more markedly in non-survivors. Lower Se levels were associated with greater tissue damage and the presence of infection and organ dysfunction. This decline in Se levels during sepsis is consistent with a mouse study in which selenoprotein P declined after administration of LPS( Reference Renko, Hofmann and Stoedter 49 ). A subsequent study found that ICU patients with low admission levels of Se were at increased risk of nosocomial pneumonia, organ system failure and mortality( Reference Forceville, Vitoux and Gauzit 50 ). During times of Se deficiency, the thyroid and brain maintain adequate levels at the expense of the immune system, which undergoes a rapid decline in availability( Reference Schomburg and Schweizer 51 ). This is problematic, given the decline in Se levels seen in patients with sepsis.
Se has been studied in mouse and in vitro models of sepsis. Se-treated mice had decreased myeloperoxidase activity (a marker of neutrophil accumulation) and decreased lung inflammation( Reference Zolali, Hamishehkar and Maleki-Dizaji 52 ). Human trials have been met with conflicting results. A multi-centred, randomised control trial involving eleven ICU in Germany in which severe sepsis or septic shock patients were randomised to either 1000 µg of sodium selenite as a 30-min bolus followed by a 14 d continuous infusion of 1000 µg v. placebo showed a mortality benefit in those receiving sodium selenite( Reference Angstwurm, Engelmann and Zimmermann 53 ). However, a more recent multi-centred randomised controlled trial involving thirty-three ICU in Germany failed to show an effect of high-dosage sodium selenite on the 28 d mortality rate of patients with severe sepsis or septic shock( Reference Bloos, Trips and Nierhaus 54 ). Both studies used the same initial bolus loading dose and daily continuous infusion. The most recent meta-analysis analysing twenty-one randomised control trials found no effect of Se on mortality( Reference Manzanares, Lemieux and Elke 55 ).
Although lower levels of Se are seen in patients with sepsis and are associated with worsening outcomes, human trials have shown mixed results. It is thus not recommended at the current time that Se be given to those patients with sepsis.
Vitamin C
Vitamin C is water-soluble and involved in numerous biosynthetic and metabolic processes. It is integral for collagen( Reference Boyera, Galey and Bernard 56 ), carnitine( Reference Hulse, Ellis and Henderson 57 ) and neurotransmitter biosynthesis( Reference Harrison and May 58 ), serves as a potent antioxidant scavenging against excess reactive oxygen species( Reference Mandl, Szarka and Banhegyi 59 ), restores other cellular antioxidants, acts as an immunomodulatory agent( Reference Carr and Maggini 60 ), and is an essential cofactor for Fe-containing enzymes( Reference Lane and Richardson 61 ). Most plants and animals synthesise ascorbic acid endogenously; however, mammalian species lack the enzyme gulonolactone oxidase, thus requiring exogenous supplementation via dietary intake and supplements( Reference Naidu 62 ). At physiological pH, ascorbate is bioavailable equally as either dehydro-l-ascorbic acid (DHA) or l-ascorbic acid (AscA). Specialised cells can take up reduced vitamin C (AscA) through Na-dependent ascorbate co-transporters (SVCT1 and SVCT2)( Reference Tsukaguchi, Tokui and Mackenzie 63 ). Most other cells take up vitamin C in its oxidised form (DHA) via facilitative GLUT( Reference Vera, Rivas and Velásquez 64 ).
In septic hosts, the immunomodulation and antioxidant activity of vitamin C complements other host responses in the multifaceted inflammatory processes, leading to sepsis-mediated multiple organ dysfunction. Vitamin C is involved in attenuating the proinflammatory and procoagulant state which induces vascular–ischaemic-induced organ injury( Reference Fisher, Seropian and Kraskauskas 65 , Reference Maeda, Hagihara and Nakata 66 ). It contributes towards the recovery of endothelial dysfunction by preventing formation of reactive oxygen species and via reductive recycling which uncouples endothelial NO synthetase( Reference Rodemeister and Biesalski 67 ). Additionally, laboratory research suggests that vitamin C reduces platelet aggregation of surface P-selectin expression( Reference Secor, Swarbreck and Ellis 68 ), attenuates hypothalamic neuronal damage( Reference Chang, Chen and Chen 69 ), may prevent cellular immunosuppression( Reference Gao, Lu and Zhai 70 ), impedes phagocyte adhesion to endothelial cells preventing phagocyte oxidative damage( Reference Koekkoek and van Zanten 71 ) and improves endogenous vasopressor synthesis( Reference Carr, Shaw and Fowler 72 ).
In critically ill patients, several investigations have demonstrated low circulating levels of vitamin C, particularly in sepsis( Reference Koekkoek and van Zanten 71 ). Ascorbate may become oxidised to ascorbyl free radical and dehydroascorbic acid, thus making ascorbate concentrations subnormal in plasma and leucocytes while plasma ascorbyl free radical concentration is elevated( Reference Wilson 73 ). Deficiency may have serious consequences and be further compounded by diminished endothelial cell uptake due to inflammatory cascade activation given that inflammatory cytokines (TNF-α, IL-1β) inhibit ascorbate uptake via down-regulation of ascorbate-specific transporters( Reference Seno, Inoue and Matsui 74 ). Plasma concentrations may become low within 24 h of onset of illness, and concentrations of <10 μmol/l are described in critically ill patients( Reference Schorah, Downing and Piripitsi 75 , Reference Berger 76 ). Low plasma concentrations are associated with inflammation, severity of organ failure, and mortality( Reference Goode, Cowley and Walker 19 , Reference Schorah, Downing and Piripitsi 75 , Reference Metnitz, Bartens and Fischer 77 – Reference De Grooth, Spoelstra-de Man and Oudemans-van Straaten 79 ).
Animal studies have supported a scientific basis for ascorbate having a beneficial and therapeutic effect in the host response to infection. Pre-existing ascorbate deficiency may decrease survival in a mouse model injected with pathogenic bacteria as animals were three times more likely to succumb to infection with introduction of Klebsiella pneumoniae ( Reference Gaut, Belaaouaj and Byun 80 ). In mouse influenza models, ascorbate deficiency has been shown to worsen lung pathology following induction of infection( Reference Li, Maeda and Beck 81 ). Additionally, animal sepsis models have demonstrated that ascorbate infusions protect against impairment of microvascular blood flow( Reference Tyml, Li and Wilson 82 ), attenuate sepsis-induced organ injury( Reference Fisher, Kraskauskas and Martin 83 , Reference Fisher, Kraskauskas and Martin 84 ) and decrease the escalation of severity of disease in combination with antimicrobials( Reference Leelahavanichkul, Somparn and Bootprapan 85 ).
While very preliminary and exploratory, human observational studies have revealed that ascorbate levels are significantly lower in septic patients and those with other aetiologies of critical illness such as severe trauma and acute respiratory distress syndrome( Reference Goode, Cowley and Walker 19 , Reference Schorah, Downing and Piripitsi 75 , Reference Metnitz, Bartens and Fischer 77 , Reference Borrelli, Roux-Lombard and Grau 78 , Reference Galley, Davies and Webster 86 ). Low plasma concentrations correlate inversely with the incidence of organ failure and markers of inflammation( Reference Borrelli, Roux-Lombard and Grau 78 ). For dosing considerations, such low serum levels are not corrected by parenteral nutrition containing the daily recommended dose of ascorbate (200 mg/d)( Reference Schorah, Downing and Piripitsi 75 , Reference Berger 76 ). This is perhaps due to the accelerated destruction of ascorbate or a depletion of total body stores during the inflammatory cascade. High doses (3 g/d) given intravenously for 3 d or more appear necessary in order to achieve normal baseline serum levels( Reference Long, Maull and Krishnan 87 ).
Preliminary-phase safety studies of high-dose ascorbate have not demonstrated any untoward pro-oxidative effects in healthy volunteers( Reference Muhlhofer, Mrosek and Schlegel 88 ). Case reports, early-phase clinical trials and retrospective human studies in septic patients have suggested that intravenous ascorbic acid infusions (either independently or in combination with thiamine and hydrocortisone) are safe, well tolerated, may reduce multi-organ failure and biomarkers of inflammation, may reduce vasopressor requirements in septic shock, and may reduce mortality in patients with severe sepsis in septic shock( Reference Marik, Khangoora and Rivera 29 , Reference Zabet, Mohammadi and Ramezani 89 – Reference Bharara, Grossman and Grinnan 91 ). However, these studies are very preliminary and limited by design and small sample size. Vitamin C may prove to be an important therapeutic option and can be considered for patients in septic shock; however, further studies are needed before it should be deemed standard of care.
Vitamin D
Vitamin D is well known for its impact on bone health through regulation of Ca–phosphate homeostasis. However, it is often forgotten that the prototypical disease of vitamin D deficiency, nutritional rickets, was originally postulated to be infectious in origin, so great was its association with pneumonia and tuberculosis( Reference Chesney 92 ). As the biochemical relationship between vitamin D and UV exposure was elucidated in the early decades of the 20th century( Reference Wolf 93 ), rachitic children with infections of the respiratory system were often treated with cod oil and sun exposure. Interest in the role of vitamin D in the human response to infection was reinvigorated in the 1980s as a greater understanding of its role in the innate and adaptive immune systems emerged.
The hormonally active form of vitamin D is calcitriol (1,25-dihydroxyvitamin D3), a metabolite of vitamin D3 derived from two sequential reactions. The primary sources of vitamin D3 are: (1) the solar UVB conversion of 7-dehydrocholesterol present in the skin; and (2) vitamin D3 directly absorbed from the gastrointestinal tract. Vitamin D3 is converted to 25-hydroxyvitamin D by vitamin D-25-hydroxylase in the liver and finally to calcitriol in the kidney and other tissues. Once converted, calcitriol binds to the vitamin D receptor (VDR), which is expressed in a wide range of cells and plays a crucial role in the optimal functioning of many organ systems and their physiological response to illness. Highlighting the importance of vitamin D status in health and response to illness, the 2011 Institute of Medicine and the Endocrine Society established minimal concentrations of>20–30 ng/dl to optimise health benefits( Reference Kempker, Han and Tangpricha 94 ). It is estimated that 1 billion individuals are vitamin D deficient, including 40–100 % of the US geriatric population( Reference Holick 95 ).
The discovery of VDR in activated CD4+, CD8+ T cells, B cells, neutrophils, macrophages and dendritic cells highlights the integral role of vitamin D in the regulation of the innate and adaptive immune system’s ability to modulate the systemic inflammatory response syndrome( Reference Baeke, Takiishi and Korf 96 , Reference Provvedine, Tsoukas and Deftos 97 ). Cathelicidin-related antimicrobial peptides (LL-37, hCAP-18) and toll-like receptors (TLR) are key components of this system and play a significant role in antimicrobial activity. Adequate concentrations of circulating calcitriol have been demonstrated to play a crucial role in LL-37 production by macrophages in bronchial epithelial cells( Reference Yim, Dhawan and Ragunath 98 ). Similarly, co-stimulation of human macrophages by calcitriol and TLR ligands appear to up-regulate the expression of VDR and result in the induction of LL-37( Reference Liu, Stenger and Li 99 ). In vitro studies have also demonstrated the anti-inflammatory effects of vitamin D by inhibiting the CD4+ Th1 cells production of cytokines IL-2, interferon and TNF( Reference Lemire 100 ). Vitamin D also enhances IL-4, IL-5 and IL-10 production of Th2 cells, in theory mitigating the deleterious effects of the pro-inflammatory state( Reference Boonstra, Barrat and Crain 101 ). Human monocytes stimulated with LPS showed dose-dependent reductions in TNF-α and tissue factor, key inflammatory molecules in sepsis( Reference Sadeghi, Wessner and Laggner 102 ).
Given the vital role that vitamin D appears to play in the innate immune response to infection, it is plausible that vitamin D levels are a determinant of risk for sepsis severity and clinical outcomes in septic shock. Indeed, vitamin D deficiency has been demonstrated in a high percentage of critically ill patients with sepsis and found to correlate with low levels of LL-37( Reference Jeng, Yamshchikov and Judd 103 , Reference Parekh, Patel and Scott 104 ). Patients presenting to an emergency department with suspected infection and a baseline 25-hydroxyvitamin D insufficiency were found to be more likely to have severe sepsis and a higher sepsis severity (sequential organ failure assessment (SOFA)>2) than infected patients with normal vitamin D levels( Reference Ginde, Camargo and Shapiro 105 ). In a large study involving more than 3000 critically ill patients, vitamin D deficiency was a significant predictor of sepsis and carried a 1·6-fold increase in mortality( Reference Moromizato, Litonjua and Braun 106 ).
Given the association with vitamin D deficiency and risk for sepsis severity and mortality, vitamin D repletion is a promising target for therapeutic intervention. In vitro models of sepsis seem to support this strategy. In murine models, pretreatment of human endothelial cells with vitamin D reduced LPS-induced production of proinflammatory cytokines( Reference Equils, Naiki and Shapiro 107 ). Similarly, sepsis-induced coagulation derangements were attenuated by the administration of 1,25-dihydroxyvitamin D3 in one study and cholecalciferol treatment 6 h post-injury reduced alveolar inflammation, cellular damage and hypoxia in another, both involving a murine model of caecal ligation and puncture( Reference Parekh, Patel and Scott 104 , Reference Moller, Laigaard and Olgaard 108 ). Clinical trials have demonstrated promise but appear to be inconclusive. Quraishi et al. ( Reference Quraishi, De Pascale and Needleman 109 ) reported an association between high-dose cholecalciferol administration and increased 25-hydroxyvitamin D and LL-37 in a cohort of septic shock ICU patients. However, a double-blind randomised trial comparing calcitriol v. placebo in sixty-seven critically ill patients with severe sepsis or septic shock failed to demonstrate an increase in plasma cathelicidin or a clear impact on immunomodulatory markers with calcitriol administration( Reference Leaf, Raed and Donnino 110 ). While not a primary outcome, supplemental calcitriol did not have an effect on mortality, or ICU or hospital length of stay. Systematic reviews of interventional trials reflect this inconsistency in results( Reference Kearns, Alvarez and Seidel 111 , Reference Langlois, Szwec and D’Aragon 112 ).
Research over the last decade has established the pluripotent regulatory role that vitamin D plays in the human response to infection( Reference Hewison 113 ). In vitro studies demonstrate the promise that interventions aimed at optimising vitamin D homeostasis can attenuate the pathophysiological cascade in septic shock models. The data are robust regarding the high prevalence of vitamin D insufficiency in the critically ill population and its relationship to critical illness outcomes including sepsis. Vitamin D supplementation is generally regarded as safe. While the demonstration of clinical efficacy for vitamin D strategies in clinical practice has proved more challenging, future investigation is warranted and forthcoming. Optimising vitamin D levels would appear to have a role in primary infection prevention for high-risk critical care populations and as an acute adjuvant therapy to mitigate the inflammatory and coagulation cascade associated with sepsis. Further clinical studies are required, and clinicians should not consider vitamin D therapy for sepsis standard of care until these trials are concluded.
Conclusion
In this modern era, mortality rates of septic shock remain unacceptably high and micronutrient resuscitative strategies may complement and enhance traditional resuscitative strategies. Additionally, with the advent of ‘Precision Health’ the identification of strategies to identify patient populations with absolute or relative micronutrient deficiency or functional pathway impedance may yield insights which could halt sepsis cascade escalation in specific hosts. For investigators, there remains an abundance of opportunities for further investigation at the molecular and biochemical levels and in human observational (i.e. biomarker) and clinical trials. If the current body of evidence is validated by higher-phase human clinical trials, sepsis micronutrient resuscitative strategies could revolutionise the current standard of care. Future trials should focus on early treatment, as inflammatory biomarkers are elevated at the most proximal part of hospital presentation( Reference Rivers, Jaehne and Nguyen 114 ). Based on all the current literature, clinicians may consider the use of thiamine in all septic patients at risk for thiamine deficiency and l-carnitine and vitamin C to those in septic shock. Future ongoing studies will lead to a clearer picture on the use of micronutrients in sepsis.
Acknowledgements
The present review received no specific grant from any funding agency, commercial or not-for-profit sectors.
J. B. B. conceived of the paper; all authors were involved in the final editing of the manuscript. Each section had a main author who wrote the first draft: J. B. B., abstract, introduction, l-carnitine, Se; C. R. W., vitamin C, conclusion; V. J., thiamine; J. E. S., vitamin D.
There are no conflicts of interest.





