Introduction
Food crises, nutritional deficiencies and associated illness affecting infants and young children are part of the history of mankind( Reference Cohen 1 ). The first records of famines date back to ancient times( Reference Dando 2 – Reference Gill 4 ), and clinical signs of severe undernutrition are mentioned in the Old Testament( 5 , 6 ). However, systematic investigations in the field of undernourishment did not exist before the second half of the 19th century, a time when advances in epidemiology, statistics and anthropometry facilitated nutritional assessments( Reference Komlos and Meermann 7 , Reference Rotberg 8 ). Concurrently, infant and young child nutrition received more attention within welfare and public health programmes in Europe and the USA( Reference McGill 9 ). After the Second World War, nutrition interventions supported by UN organisations and international non-governmental organisations were increasingly implemented in developing countries, aiming to combat nutritional deficiencies, a major cause of under-five morbidity and mortality. In 2016, an estimated 155 million (22·9 %) children under 5 years were stunted, and 52 million (7·7 %) were wasted including 16·9 million (2·5 %) with severe wasting. Over 41 million children (6 %) globally were overweight or obese( 10 ).
In many programmes for the prevention and treatment of childhood undernutrition, dairy products constitute key components. There is a growing body of evidence supporting the positive effects of milk proteins on the linear growth of healthy children and catch-up growth during recovery from undernutrition( Reference Manary, Callaghan and Singh 11 , Reference Yackobovitch-Gavan, Phillip and Gat-Yablonski 12 ). While potential mechanisms and responsible components of dairy products influencing body composition as well as weight and height gain were extensively studied during recent years, many questions remain unanswered( Reference Yackobovitch-Gavan, Phillip and Gat-Yablonski 12 ).
In the area of infant feeding and childhood undernutrition previous reviews have described developments chronologically( Reference Quandt 13 – Reference Hansen 17 ). Other historical reviews focused on breast-feeding, complementary feeding and various types of nutritional deficiencies( Reference Fildes 15 – Reference Papastavrou, Genitsaridi and Komodiki 28 ). Some were confined to distinct time periods or geographical contexts( Reference Obladen 21 , Reference Weaver 29 – Reference Heikens and Manary 37 ). Similarly, a great number of reviews regarding milk product utilisation( Reference Vernon 38 – Reference Valenze 43 ) addressed very specific aspects of prevention and treatment of childhood undernutrition.
The purpose of the present historical review is to address the following questions:
(1) How did milk-based strategies to combat undernutrition in under-5-year-old children evolve over time?
(2) What were the impacts of medical doctrines, research findings and technical innovations?
Terms used in this review
The term ‘milk/dairy products’ refers to items produced from, or containing, milk of mammals, primarily cattle. These products include infant formula, yoghurt, cheese, condensed milk, skimmed milk, whey protein concentrate, milk-based therapeutic foods, etc., whereas the term ‘milk’ usually refers to bovine milk.
While the term ‘malnutrition’ includes forms of overnutrition (i.e. overweight and obesity), the present review focuses on states of undernutrition in children under 5 years, including underweight, wasting, stunting, micronutrient deficiencies and low birth weight( Reference Corvalan, Dangour and Uauy 44 ). In this article, the term ‘undernutrition’ is used, unless other terms such as kwashiorkor, marasmus, protein–energy malnutrition, severe acute malnutrition (SAM) and moderate acute malnutrition (MAM) were used in publications of that time.
In Table 1 ( Reference Hansen 17 , Reference Waterlow 23 , Reference Allen 25 , Reference Scherbaum 45 – Reference Grobler-Tanner and Collins 69 ), medical terms used for specific forms of undernutrition during various time periods are summarised.
Table 1 Terms used for various forms of undernutrition/malnutrition in historyFootnote *
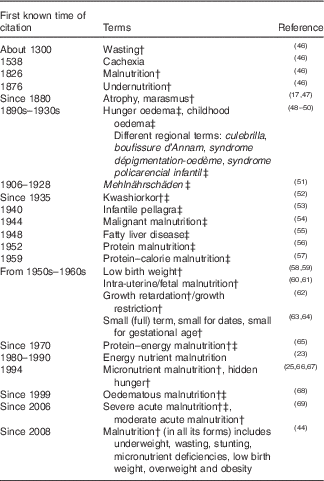
* Modified from Scherbaum( Reference Scherbaum 45 ).
† Term still used today.
‡ Oedematous forms of undernutrition.
Use of milk products in infant and young child feeding until the 20th century
Findings of fatty residues in Neolithic feeding vessels in Europe suggest that products from animal milk were introduced for feeding young children more than 7000 years ago( Reference Lacaille 70 ). It is assumed that from very early time preservation of milk was achieved through heating, fermentation and the manufacture of yoghurt, cheese and butter( Reference Vernon 38 , Reference Tamime 71 – Reference Salque, Bogucki and Pyzel 73 ). In ancient civilisations of the Mediterranean region, the Middle East and India breast-feeding was viewed as essential to preserve life and was an obligation of mothers( Reference Fildes 15 , Reference Papastavrou, Genitsaridi and Komodiki 28 ). When breast milk could not be provided by mothers or wet nurses, animal milk was offered to young children, sometimes directly from the udder of animals( Reference Castilho and Barros Filho 16 ). Milk was seen as more than solely a source of nutrition. It was often regarded as a heavenly elixir reflecting fertility and the nurturing mother–infant relationship while its whiteness was a symbol of goodness and purity( Reference Vernon 38 , Reference Obladen 40 ). Since antiquity, differences in the composition of milk between mammalian species were described and certain qualities were attributed to particular types of animal milk. For example, bovine milk was used as a remedy against specific illnesses during the Roman Empire( Reference Obladen 40 ). Concerning infant feeding, animal milk has been used for centuries as the main component of artificial foods including as a substitute for colostrum after delivery( Reference Castilho and Barros Filho 16 ).
In 1610, O. Gaebelkhovern highlighted that children fed with diluted cows’ milk combined with cereal preparations thrived better than those fed solely with unmodified cows’ milk( Reference Fildes 15 , Reference Radbill 74 ). This observation was endorsed in 1838 by J. F. Simon after his discovery that cows’ milk contains more protein and less carbohydrate than human milk( Reference Cone 75 , Reference Schuman 76 ). His findings led to a variety of mixtures with cereal preparations, diluted cows’ milk( Reference Fildes 15 , Reference Grabmayr and Scherbaum 77 ) and often enrichments with sugar and cream( Reference Barness 78 ). In 1884, P. Biedert in Germany and C. D. Meigs in the USA made a precise comparison of the nutrient contents in cows’ milk and human breast milk. Based on their metabolic studies in the 1890s, Otto Heubner and M. Rubner calculated the daily energy requirements, using calorimetric methods, for healthy and undernourished infants and young children( Reference Heubner 79 ). At the same time, T. M. Rotch published a method for calculating the precise proportions of carbohydrates, proteins and fats required to substitute diluted cows’ milk for human milk( Reference Mepham 80 ). This so-called ‘percentage method’ as well as the ‘calorie method’ introduced by H. Finkelstein were too complicated for the production of artificial formula at the household level( Reference Obladen 40 ). On the basis of these research findings, the formula industry used the opportunity to develop artificial infant formulas( Reference Mepham 80 – Reference Wood 82 ).
For centuries, contamination of milk during production, transport and dilution with polluted water has significantly contributed to the high morbidity and mortality of artificially fed infants( Reference Obladen 83 ). This feeding practice was particularly common among mothers of lower classes employed in factories, who were forced to wean their babies shortly after delivery( Reference Mepham 80 , Reference Wickes 84 ). Besides general improvements in hygiene, by the middle of the 19th century important innovations took place that reduced the risk of milk-borne diseases in Europe and the USA. First, the invention of evaporation enabled the production of condensed milk. Second were the discovery and utilisation of pasteurisation techniques( Reference Obladen 21 ). The claims of ‘clean milk movements’ led to the supply of, often subsidised, ‘clean’ milk in special dispensaries, milk depots and infant welfare centres. Mandatory pasteurisation laws were adopted in many countries( Reference Lee 85 – Reference Bloomfield and Scott 87 ).
The introduction of milk pasteurisation was a milestone in the history of public health. Like other improvements in infection protection, these measures were built on study results in the field of infection epidemiology by J. Snow( Reference Winkelstein 88 , Reference Paneth and Fine 89 ) and findings in bacteriological research by R. Koch and L. Pasteur in the middle of the 19th century( Reference Exner, Hartemann and Kistemann 86 , Reference Bloomfield and Scott 87 ). However, these advances facilitated the spread of the ‘germ theory’ which influenced medical thinking that the causes of diseases can be ascribed mainly to microbes( Reference Carter 90 ). Moreover, the ‘germ theory’ when applied to diseases like beriberi( Reference Carter 90 , Reference Bennett 91 ) was a general barrier to the recognition of deficiency diseases( Reference Ihde and Becker 92 ). Only in the second decade of the 20th century did evidence of specific micronutrient deficiencies lead to the aetiological concept of ‘deficiencies’( Reference Funk 93 ).
At the end of the 19th century, it was recognised that young children who were fed with unfortified pasteurised milk, condensed milk or industrially produced infant formula( Reference Rajakumar 94 ) were developing infantile scurvy. Based on the ‘germ theory’, the aetiology of chronic poisoning by absorption of ptomaine toxin, a waste product of bacteria, was suggested. In 1914, the paediatrician A. Hess proved by experiments that through pasteurisation antiscorbutic properties of milk are destroyed, which could be prevented by supplementing fresh fruit or vegetable juices when infants received these formulas( Reference Rajakumar 94 ).
Use of milk products in nutrition programmes in the first half of the 20th century
At the beginning of the 20th century, the German paediatricians A. Czerny and A. Keller suggested that overfeeding with cows’ milk to young children leads to ‘Milchnährschäden’ with symptoms of dyspepsia and failure to thrive. Similarly, a monotonous diet containing mainly cereal flour was suspected to be the main cause of ‘Mehlnährschäden’ characterised by undernutrition with oedema, thought to be secondary to protein deficiency( Reference Czerny and Keller 51 , Reference Kraus, Meyer and Minkowski 95 , Reference Salge 96 ). At the same time, the concept of an alimentary toxicosis was proposed by H. Finkelstein. He suspected that the degradation of certain alimentary substrates, through fermentation of carbohydrates, produced toxins in the immature gut of infants leading to food intolerance with diarrhoea and weight faltering( Reference Finkelstein 97 ). The presumed aetiology of the unwholesome effects of an inappropriate composition of children’s diets spread worldwide. Attention was distracted from effective measures against essential causes of infant gastroenteritis, namely fundamental improvements in hygiene and promotion of breast-feeding. During the following decades, investigations failed to detect any of the postulated toxins. However, a variety of therapeutic milk-based preparations was developed, for example Finkelstein’s ‘protein milk’ or modified buttermilk( Reference Nützenadel 98 ).
In countries, such as England, cows’ milk played a particular role in charitable feeding at the beginning of the 20th century and was most commonly provided to debilitated and severely undernourished children, often together with cod liver oil( Reference Atkins 99 ). In the 1920s, when a growing number of ‘accessory factors’ (vitamins) were discovered, milk was increasingly considered as a ‘complete food’ and a special nurturing medium for young children( Reference Pollock 100 , Reference Allen and Dror 101 ). During that time, intervention studies in the USA and Britain revealed a positive effect of supplementary milk feeding on the nutritional status of school-age children( Reference McCollum 102 – Reference Leighton and Clark 105 ). These study results contributed to the expansion of supplementary milk feeding programmes in Britain during the 1930s. Due to major methodological constraints, the results of these studies were questioned by some authors and the influence of the dairy industry on nutrition policies was critically debated( Reference Pollock 100 , 106 , Reference Atkins 107 ).
The high prevalence of child undernutrition between both World Wars led to relief programmes in Austria, Germany, Poland, Russia and other countries, delivered by organisations including Save the Children Fund and the support of philanthropists( Reference Sellick 108 , Reference Roberts 109 ). In 1922, Russia, food aid containing milk-based foods, such as mixtures of canned milk with maize, sugar and fats, was implemented by the American Relief Administration for Russian children( Reference Rhodes 110 ). In Germany, the ‘Moro Brei’, a gruel made of whole milk, butter, flour and sugar was frequently offered to undernourished children. To safeguard the quality of these preparations under adequate hygienic conditions, special milk kitchens were established in hospitals( Reference Nützenadel 98 ).
Nutritional interventions and protein-role controversies
Experiences in Europe and the USA influenced interventions promoted in overseas territories. Tinned condensed milk was used by the paediatrician Cicely Williams in combination with malt and cod liver oil to treat children suffering from kwashiorkor in the former British colonies on the ‘Gold Coast’ of West Africa in the 1930s( Reference Williams 52 , Reference Williams 111 ). Based on her observations of affected children who were fed a monotonous maize diet, she presumed that protein deficiency was the main cause of oedematous forms of undernutrition( Reference Williams 111 ). Her positive view regarding the use of condensed milk in infant feeding rapidly changed after she was transferred to Malaya( Reference Stanton 112 ) where this product was being used by mothers as a breast milk substitute. As early as 1880 sweetened condensed milk was advertised as ‘…the food par excellence for delicate infants’( Reference Richter 113 ) and was advocated by colonial doctors. Many mothers were convinced by female milk industry employees, dressed as nurses, that this milk product was the best replacement for their own breast milk. In 1939, in her famous speech entitled ‘Milk and Murder’ held in the Rotary Club in Singapore, Williams named and shamed this practice and its consequences, manifesting in diarrhoea, marasmus and death( Reference Williams 114 ).
During the following decades undernutrition remained a major public health problem in many parts of the world. While protein deficiency was widely regarded as a major cause of oedematous forms of undernutrition, milk was seen as the best source of protein( Reference Brock and Autret 56 , Reference Gopalan 115 ). Since animal protein was expensive shortly after the Second World War, trials were conducted using plant proteins such as soya, bananas, plantains and maize flour( Reference Dean 116 , Reference Dean 117 ). However, milk-based diets showed the best results, particularly for treating severely undernourished children( Reference Dean 117 – Reference Spies, Dreizen and Snodgrasse 120 ). From the 1950s onward, supplementation with skimmed milk was increasingly practised in nutrition programmes delivered by UNICEF aiming to control ‘protein malnutrition’. However, milk powder surpluses decreased in industrialised countries, and achievement of significant increases in local milk production in many overseas regions appeared to be unrealistic( Reference Brock and Autret 56 , Reference Carpenter 121 ). In 1955, a Protein Advisory Group was initiated by the WHO and efforts were made to investigate alternative non-milk foods to combat a supposed worldwide ‘protein gap’( 122 ). These efforts included the production of ‘protein rich food mixtures’ based on fish, flour, soya, cottonseed, groundnuts, sesame or coconuts. However, many of these innovative approaches were halted due to difficulties in food technology, food safety considerations and high costs that made these products unaffordable for poor populations( Reference Carpenter 121 ).
Until the foundation of the World Food Programme in the early 1960s, surpluses primarily of cereals from industrialised countries were distributed to countries suffering from humanitarian crises. Subsequently, fortified blended foods, consisting of maize or wheat, vegetable oil and sugar, were introduced in supplementary feeding programmes. Skimmed milk and soya flour were added as protein sources reflecting the ‘protein–calorie deficiency’ theory. When US milk surpluses were exhausted and evidence revealed ineffective and unsafe use of milk powder in community feeding programmes, this food aid compound was gradually replaced by maize–soya or wheat–soya blends( Reference Marchione 123 , Reference Fleige, Moore and Garlick 124 ). By the 1980s, the daily protein requirements for children were gradually reduced( 125 – 127 ), and the dogma of protein deficiency was questioned and refuted by research( Reference Scherbaum and Furst 128 – Reference Golden 130 ). Early observations revealed that undernourished children often received too few meals a day and primarily bulky foods with low energy density( Reference Welbourne 131 , Reference Welbourne 132 ). The energy content of complementary foods was readdressed. With respect to oedematous forms of undernutrition, the aetiological concept shifted from ‘protein malnutrition’ in the 1950s( Reference Brock and Autret 56 ) to ‘protein–calorie malnutrition’ in 1959( Reference Jelliffe 57 ) to ‘protein–energy malnutrition’ in the 1970s( 65 ). In addition, the theory of a multifactorial aetiology developed including the potential impact of infections, aflatoxins as well as micronutrient deficiencies, free radicals and most recently alterations of the intestinal microbiome( Reference Scherbaum and Furst 128 , Reference Hendrickse, Coulter and Lamplugh 133 – Reference Kane, Dinh and Ward 136 ). In the mid-1970s, the focus on a worldwide ‘protein gap’ faded, but during the next decades, expert committees of the FAO and WHO continued to address protein and amino acid requirements in human nutrition.
Increasing awareness and the ‘rethinking protein’ with respect to childhood undernutrition are a new development( 126 , 127 , Reference Uauy 137 – Reference Semba 140 ). A large proportion of children in low-income countries depend on low-protein diets. These children are frequently affected by chronic energy deficits, repeated infections and stunting, so research has explored the role of protein quality determined by its digestibility and bioavailability of essential amino acids( Reference Manary, Callaghan and Singh 11 , 138 , Reference Ghosh 141 , Reference Ghosh, Suri and Uauy 142 ). A recent study in Malawi demonstrated a correlation between a high prevalence of stunting and reduced levels of circulating essential amino acids among children below 5 years of age( Reference Uauy 137 , Reference Ghosh, Suri and Uauy 142 – Reference Uauy, Suri and Ghosh 145 ).
Development of milk products used for dietary interventions
Milk has long been recognised as a well-balanced source of energy with numerous essential nutrients( Reference Yackobovitch-Gavan, Phillip and Gat-Yablonski 12 , Reference Haug, Hostmark and Harstad 146 ) playing a key role in treating childhood undernutrition both in industrialised and in developing countries( Reference Weaver, Wijesinha-Bettoni and McMahon 147 ).
Milk is known to contain high-quality protein with all essential amino acids including lysine which is often deficient in traditional cereal-based diets of agriculturist populations( Reference Sadler, Kerven and Calo 42 , Reference Wijesinha-Bettoni and Burlingame 148 ). The two main fractions of milk are the water-soluble ‘whey’ protein and insoluble ‘casein’ protein. Various authors consider milk to be the best protein source according to the essential and protein-digestibility amino acid scores( Reference Reeds, Schaafsma and Tome 149 – Reference Pereira 151 ). Milk protein positively affects linear growth in healthy children and there is growing evidence of similar effects on recovery from childhood undernutrition( Reference Yackobovitch-Gavan, Phillip and Gat-Yablonski 12 , Reference Gat-Yablonski, Yackobovitch-Gavan and Phillip 152 ). The beneficial effects of milk protein, commonly in combination with micronutrient supplementation, were demonstrated in a dose–response relationship on catch-up height and weight gain( Reference Oakley, Reinking and Sandige 153 , Reference Batra, Schlossman and Balan 154 ). Moreover, high-quality milk proteins contribute to effective immune functions by increasing acute-phase protein synthesis in response to infections which often accompany SAM( Reference Manary, Callaghan and Singh 11 , Reference Yackobovitch-Gavan, Phillip and Gat-Yablonski 12 , Reference Ghosh 141 ).
The specific effectiveness of high-quality whey protein in the treatment of moderately wasted children (age 6–59 months) was highlighted in a recent intervention study in Malawi and Mozambique. Supplementation with whey-based products resulted in better recovery and growth rates than did supplementation with products based on soya, even though the whey-based supplement provided 33 % less total protein and 8 % less energy than the soya-based product( Reference Stobaugh, Ryan and Kennedy 155 ).
Regarding the carbohydrate content of milk, the disaccharide lactose is known to enhance Ca absorption and, like specific oligosaccharides released from milk glycoproteins, induces prebiotic effects on the gut microbiome contributing to enhanced efficiency of food utilisation in the intestine( Reference Yackobovitch-Gavan, Phillip and Gat-Yablonski 12 , Reference Haug, Hostmark and Harstad 146 , Reference Grenov, Briend and Sangild 156 , Reference Karav, Le Parc and Leite Nobrega de Moura Bell 157 ).
Milk supplies key micronutrients like Ca, P, Se, Mg, Zn and vitamins A, D, E and B, without the antinutrients, such as phytates and oxalates( Reference Pereira 151 ). In addition, milk contains bioactive compounds exhibiting a wide variety of physiological functionalities, including mineral transport and growth-promoting activities( Reference Park and Nam 158 ).
Since 1970, in very severe cases of undernutrition, milk was seen as an excellent vehicle for micronutrient fortification and was valued for its liquid form, enabling nasogastric tube feeding( Reference Ashworth 159 – Reference Ashworth, Jackson and Khanum 161 ). On the basis of clinical experience, past research findings and new knowledge about the role of micronutrients, specific therapeutic milk formulas (F-75, F-100) were created. These contain relatively low concentrations of protein and a mixture of specific micronutrients( Reference Briend and Golden 162 ). Treatment regimens using these milk formulas for the management of severe undernutrition( Reference Ashworth, Jackson and Khanum 161 , Reference Ashworth and Burgess 163 ) were developed and published by the WHO in 1999( 68 ).
During the same year, a paste made of groundnut butter, milk powder, vegetable oil and sugar, fortified with the same mix of micronutrients, was tested in a pilot study of marasmic children treated in a therapeutic feeding centre in Chad( Reference Briend, Lacsala and Prudhon 164 ). The effectiveness of this so-called ‘ready-to-use therapeutic food’ (RUTF) was demonstrated in Sub-Saharan Africa( Reference Collins and Sadler 165 , 166 ). The milk-powder in F-100 was partially replaced by groundnut paste and changed from liquid to a spread, making feasible the community management of children with SAM( Reference Collins and Sadler 165 , 167 ), accepted internationally in 2007( 166 ). RUTF do not require cooking and their low moisture content reduces the risk of bacteria and mould growth, allowing for a long shelf life, even without refrigeration.
According to the WHO, there is sufficient evidence of the efficacy of milk products in the dietary management of SAM( 68 , 167 ). This applies for F-75 and F-100 therapeutic milk formula for the hospital-based treatment of cases with complications and RUTF for the community-based management of SAM without complications( Reference Collins, Dent and Binns 168 ).
Growing commercialisation of ready-to-use foods
The commercial marketing of costly RUTF, patented and primarily produced in industrialised countries, made its usefulness debatable( Reference Latham, Jonsson and Sterken 169 , Reference Greiner 170 ). This stimulated the development of locally produced therapeutic food, acceptable and cost-effective in most trials( Reference Sandige, Ndekha and Briend 171 – Reference Weber, Ryan and Tandon 173 ). During the last decade, research shifted to the management of MAM, to prevent the development of severe forms, which is more expensive and management time consuming( Reference Purwestri, Scherbaum and Inayati 174 ).
Consequently, improved fortified blended foods were developed to provide the energy and nutrient requirements of infants and young children during disasters( Reference Fleige, Moore and Garlick 124 ). Ready-to-use supplementary foods (RUSF), with a higher energy density and adjusted micronutrient compositions, were developed for moderately wasted children, as well as pregnant and lactating women( Reference Fleige, Moore and Garlick 124 , 175 – Reference de Pee and Bloem 177 ). The inclusion of milk protein in RUSF appears beneficial in children recovering from MAM( Reference Stobaugh, Ryan and Kennedy 155 ). Two recent intervention studies in Guinea-Bissau demonstrated that RUSF with a higher dairy protein content (33 %) were superior to RUSF with a lower content (15 %) in the community-based management of undernourished preschool children and mothers( Reference Batra, Schlossman and Balan 154 , Reference Schlossman, Brown and Batra 178 ).
In 2009, the International Lipid-Based Nutrient Supplements project aimed to develop lipid-based nutrient supplements (LNS) for the prevention of undernutrition in food-insecure settings( 175 ). LNS are special types of RUSF with varying energy densities and micronutrient concentrations, with or without small amounts of milk products. As these industrially manufactured items need to be offered only in small doses, they were added to general food rations for at-risk populations( 175 , Reference Chaparro and Dewey 179 ).
Strategies to reduce the costs of milk-based ready-to-use foods
The major constraint of using milk-based ready-to-use foods is the high cost. With respect to standard RUTF, of the total expenses per child cured from SAM (US$ 70–200), about half is spent for the therapeutic product alone( Reference Greiner 170 , 180 ). More than half the cost of the therapeutic formulation is due to the milk powder( Reference Purwestri, Scherbaum and Inayati 174 , Reference Manary 181 ).
An attempt to reduce the milk powder content by 25 % in standard RUTF by replacing 15 % with soya showed that the product with only 10 % milk powder was clinically less effective in the treatment of SAM, in weight gain and recovery rates. While both formulations had nearly identical nutrient contents, the content of milk protein, of antinutrients or the impact of unidentified beneficial factors associated with milk supplementation might explain the difference in effectiveness( Reference Oakley, Reinking and Sandige 153 ).
To reduce the costs of milk-based ready-to-use foods, projects were implemented aiming to replace the skimmed milk content with whey protein concentrate. Whey protein concentrate (34 %) is about 25–33 % cheaper and can be generated as a surplus product from cheese manufacturing( Reference Wijesinha-Bettoni and Burlingame 148 ). Recent interventions with products based on whey containing high-quality protein, high levels of lactose, micronutrients and bioactive factors have yielded promising results in the dietary management of MAM and SAM( Reference Hoppe, Andersen and Jacobsen 182 – Reference Bahwere, Banda and Sadler 184 ).
As many countries in Africa and Asia largely rely on imported dairy products, efforts were made during the last 10 years to develop suitable milk-free alternatives based on locally available foods at relatively low cost( Reference Michaelsen, Hoppe and Roos 185 , Reference Lhotska, Scherbaum and Bellows 186 ). These ready-to-use-food formulations commonly contain cereal flours, pulses, nuts and/or seeds, and vegetable oil and sugar which are often supplemented with a mineral–vitamin premix. While the acceptability of these products has been reported to be generally good( Reference Purwestri, Scherbaum and Inayati 187 , Reference Purwestri, Scherbaum and Inayati 188 ), concern has been raised about the higher content of fibres and antinutrients such as phytates in milk-free formulations, which can impair the bioavailability of micronutrients including Fe and Zn( Reference Kana Sop, Gouado and Mananga 189 ). Processing such as dehusking, soaking, roasting, malting, germination and fermentation have been used to lower the anti-nutrient content( Reference Hotz and Gibson 190 ). Apart from the cost and acceptability considerations of alternative formulations, the optimisation of protein quality is particularly important in the development of milk-free products. Linear programming can contribute to choosing the appropriate ingredients such as foods of animal origin or best combinations of locally available plant proteins( Reference Weber and Callaghan 191 ). The palatability and taste of the improved recipes must be evaluated for acceptance by the target groups( Reference Scherbaum, Purwestri and Stuetz 192 ).
During the last decade, research teams particularly in countries of Sub-Saharan Africa evaluated the efficacy of therapeutic formulations without dairy ingredients compared with milk-based products, including standard RUTF and preparations containing whey. Regarding a formulation containing whey protein concentrate, an equally effective alternative to standard RUTF was demonstrated in the treatment of SAM with lower costs( Reference Bahwere, Banda and Sadler 184 ).
Compared with the efficacy of milk-based products, certain milk-free formulations have been shown to be equivalent in the management of MAM( Reference LaGrone, Trehan and Meuli 183 , Reference Scherbaum, Purwestri and Stuetz 192 – Reference Nikiema, Huybregts and Kolsteren 194 ), whereas in other studies, these formulations were less effective regarding the treatment of MAM( Reference Stobaugh, Ryan and Kennedy 155 , Reference Ackatia-Armah, McDonald and Doumbia 195 – Reference Patel, Sandige and Ndekha 200 ) as well as SAM( Reference Irena, Bahwere and Owino 201 ). While a reduced efficacy of preparations without milk was generally most pronounced in children below the age of 2 years, it has been suggested that milk-free products should be used preferentially in the treatment of undernourished children older than 2 years, whereas the younger age group may depend more on products containing dairy products, especially if breast-feeding has been terminated( Reference Irena, Bahwere and Owino 201 , Reference Bahwere, Balaluka and Wells 202 ). Very recently, a study in Malawi showed that a milk-free formulation containing soya, maize and sorghum, enriched with crystalline amino acids, was as efficacious as standard RUTF with respect to recovery rates of SAM children aged 6–23 months and 24–59 months. Moreover, this milk-free formulation was even better at correcting Fe-deficiency anaemia( Reference Bahwere, Akomo and Mwale 203 ).
The costs of dietary regimens currently used in the treatment of MAM were recently summarised by Suri et al. ( Reference Suri, Moorthy and Rosenberg 39 ). There remains a need to evaluate the cost-effectiveness of treatment by costs per impact or effect( Reference Rosenberg, Rogers and Webb 204 ). The required time period of treatment and nutritional advantages of certain ingredients, the palatability of products and acceptability of interventions and the compliance of the target group should be considered in future programmes( Reference Scherbaum, Purwestri and Stuetz 192 , Reference DiRienzo 205 ).
Limitations of current studies, knowledge gaps and research needs
Appraisals of the beneficial role of milk supplementation are often compromised by failing to meet internationally agreed criteria of study design and reporting( Reference Schulz, Altman and Moher 206 , 207 ) and lack relevant study details. Precise information on the amount of dairy products, other sources of protein, the amount of supplementary food offered and actually consumed by the target group, variations in compliance, and the potential impact of educational interventions are lacking( Reference DiRienzo 205 , Reference Inayati, Scherbaum and Purwestri 208 ). Similarly, the validity of results from clinical trials could be enhanced by comparing isoenergetic and isoenergetic plus isonitrogenous dietary conditions, but this has been accomplished in very few studies( Reference Noriega and Lindshield 209 ).
Additional information is required about the intensity and long-term duration of breast-feeding, the quality of complementary foods as well as family foods including seasonal nutritional insecurities( Reference Scherbaum and Srour 210 ).
There is still no scientific evidence about the minimum amount of milk protein required to exert an adequate effect on weight gain and growth among children of different age groups( 68 ), while the impact of other sources of protein and other beneficial compounds needs to be considered( Reference Suri, Moorthy and Rosenberg 39 ).
As most estimations of protein, amino acid and other nutrients are based on measurements among healthy individuals, research on nutrient requirements for undernourished children is needed to improve the composition of therapeutic diets to achieve adequate catch-up growth during different stages of treatment, rehabilitation and to minimise potential adverse effects in later life( Reference DiRienzo 205 , Reference Pencharz, Jahoor and Kurpad 211 , Reference Singhal 212 ).
Regarding the effectiveness of supplementary feeding programmes, there is still no conclusive evidence with respect to the potential of LNS to achieve adequate weight gain( Reference Thakwalakwa, Ashorn and Jawati 213 ) and certain developmental outcomes, including the prevention of growth faltering of children( Reference Phuka, Gladstone and Maleta 214 – Reference Mangani, Maleta and Phuka 217 ). A Cochrane analysis in 2013 showed no proven benefits of LNS compared with other blended and less costly foods such as fortified maize–soya blend( Reference Lazzerini, Rubert and Pani 218 ).
Challenges regarding milk-based dietary interventions
The relatively low Fe content and bioavailability of Fe in bovine milk is a particular drawback in offering cows’ milk to infants during the complementary feeding period( 219 , Reference Dror and Allen 220 ). In 1992, the American Academy of Pediatrics recommended avoiding whole cows’ milk before 1 year of age due to its high renal solute content, which places small children at increased risk of dehydration under conditions of water stress. In addition, the risk of occult intestinal bleeding, which can be prevented by using heat-treated cows’ milk( Reference Fomon, Ziegler and Nelson 221 , Reference Dewey 222 ), was taken into consideration( Reference Allen and Dror 101 , 223 – Reference Ziegler 226 ). Regarding supplementation of Fe, due to its critical role in catalysing free radical oxidation and susceptibility to infectious diseases, it is generally not recommended to give Fe during the initial stabilisation phase of SAM( 68 , Reference Ashworth and Burgess 163 ). Children treated with nutrient-dense RUTF need to drink sufficient extra water, challenging in many settings with inadequate safe water supplies( Reference Greiner 170 ).
Clearly, conditions of undernutrition in the first 2 years of life, such as stunting, severe wasting and intra-uterine growth restriction, are known to cause considerable harm regarding the health and development of the child( Reference Black, Bhutta and Bryce 227 ). However, there are concerns that excessive weight gain during and after rehabilitation may be associated with adverse effects on long-term health( Reference Singhal 212 , Reference Weaver 228 , Reference Bhutta, Ahmed and Black 229 ). These concerns are based on results from observational studies suggesting that accelerated weight gain and growth in early life enhance the risk for developing obesity and cardiometabolic diseases( Reference Singhal 212 , Reference Eid 230 – Reference Marinkovic, Toemen and Kruithof 233 ). As increased growth velocity has been observed among infants who were never or only briefly breast-fed, this effect was explained by the higher milk protein content of artificial infant formulas compared with human breast milk( Reference Mameli, Mazzantini and Zuccotti 234 – Reference Koletzko, von Kries and Closa 237 ).
While there is some controversy concerning obesogenic influences of high intake of milk protein in early life( Reference Lu, Xun and Wan 238 ), critical protein levels in different age groups and contexts have yet to be established( Reference Singhal 212 ) and pathogenic mechanisms like the suggested induction of insulin and insulin-like growth factor-1 are still hypothetical( Reference Hoppe, Molgaard and Juul 239 – Reference Socha, Grote and Gruszfeld 241 ).
Apart from the need to support long-term protective effects of breast-feeding against obesity, various gaps in research on the ‘growth acceleration hypothesis’ must be addressed( Reference Singhal 232 ). The short-term benefits of dietary interventions resulting in rapid catch-up weight and growth should be counterbalanced with potential risks of non-communicable diseases in later life( Reference Singhal 212 , Reference Jain and Singhal 242 ). There is some evidence that the long-term effects of whey protein and casein on linear growth are similar; in contrast, whey protein induces less weight gain as compared with casein( Reference Lebenthal, Yackobovitch-Gavan and Lazar 243 – Reference Yackobovitch-Gavan, Lebenthal and Lazar 245 ). Consequently, long-term obesogenic complications of catch-up growth may be diminished by the use of whey-based diets( Reference Yackobovitch-Gavan, Phillip and Gat-Yablonski 12 , Reference Gat-Yablonski, Yackobovitch-Gavan and Phillip 152 ).
The implementation of milk-based interventions in regions with low milk consumption is problematic. Areas with a higher prevalence of lactase deficiency may limit caregiver acceptance of milk in young child feeding( Reference Scherbaum, Purwestri and Stuetz 192 ). As lactase deficiency usually manifests itself among children older than 5 years( Reference Vandenplas 246 ), the American Academy of Pediatrics stated in 1978 that ‘…it would be inappropriate to discourage supplemental milk feeding programs targeted at children on the basis of primary lactose intolerance’( 247 ). Milk-based diets have not been shown to cause major clinical concern, neither in young children with diarrhoea nor in the treatment of child undernutrition( Reference Solomons, Torun and Caballero 248 – Reference Brown, Peerson and Fontaine 251 ). In fact, there is evidence that the prebiotic effects of lactose facilitate the absorption of minerals( Reference Grenov, Briend and Sangild 156 , Reference Ziegler and Fomon 252 ). It has been suggested that undernourished children with secondary lactase deficiency caused by environmental enteropathy and diarrhoeal diseases might benefit from products with reduced lactose content( Reference Grenov, Briend and Sangild 156 ). A meta-analysis of studies in low- and middle-income countries among children with acute diarrhoea demonstrated that liquid feeds with reduced lactose content such as yoghurt were not superior to those containing lactose, whereas liquid lactose-free diets reduced both the duration and risk of treatment failure( Reference Gaffey, Wazny and Bassani 253 ).
For many years the distribution of powdered milk in emergency situations has been a major challenge, particularly when the product has to be reconstituted with unsafe water( Reference Scherbaum 254 – Reference Michaelsen, Nielsen and Roos 256 ) or when milk powder is contaminated with Enterobacter sakazakii ( Reference Jacobs, Braun and Hammer 257 ). The policy was adopted that humanitarian organisations should not distribute milk powder as take-home rations( Reference Mourey 258 ). Similarly, it has been recognised that the high renal solute load of drinks prepared from skimmed milk powder can be particularly harmful for undernourished infants whose maximum renal concentrating capacity is significantly reduced( Reference Ziegler 226 , Reference Michaelsen, Nielsen and Roos 256 , Reference Benabe and Martinez-Maldonado 259 ).
The current focus on ‘product-based’ approaches to combating childhood undernutrition is seen critically by a variety of authors who point out that ready-to-use foods tend to consume most of the financial resources and are detracting investments from long-term and sustainable programmes( Reference Greiner 170 , Reference Mason and Margetts 260 ). Comprehensive interventions are needed to address the multifactorial causes of childhood undernutrition, already outlined 30 years ago( Reference McLellan 135 , Reference Latham, Jonsson and Sterken 169 , Reference Taylor and Taylor 261 , 262 ). Due to the specific requirements of undernourished children of different age groups living in various settings, appropriate dietary management cannot be met with industrial products designed in the sense of ‘one size that fits all’( Reference Krumbein, Scherbaum and Biesalski 172 , Reference Schweitzer 263 ).
Feeding commercial foods aiming to prevent MAM during a particularly sensitive time period of early child development might shape the taste preferences of young children towards food items other than those locally available( Reference Greiner 170 , Reference Mennella 264 ). Although recent studies did not confirm a decrease in breast-feeding rates and intake of commonly consumed foods during home fortification of complementary foods with LNS( Reference Galpin, Thakwalakwa and Phuka 265 – Reference Flax, Siega-Riz and Reinhart 268 ), a negative impact under non-research conditions and uncontrolled promotion of products can never be ruled out( Reference Greiner 170 ).
Although breast-feeding offers children and mothers unrivalled health benefits, worldwide deficits in breast-feeding promotion and support are a ‘missed opportunity for global health’( 269 ). Aggressive marketing by the formula industry and violations of The International Code of Marketing of Breast-milk Substitutes undermine breast-feeding promotion and contribute to the decline of breast-feeding rates( Reference Barennes, Slesak and Goyet 270 ) with immense health consequences, named ‘commerciogenic malnutrition’ by the paediatrician D. Jellife in 1972( Reference Jelliffe 271 ). A recent study in Laos has shown that parents misperceive coffee creamer products as suitable for infant feeding and use them as breast milk substitutes( Reference Barennes, Andriatahina and Latthaphasavang 272 ).
Conclusion
The present historical review describes the changing role and development of milk products in infant and young child feeding and in the dietary management of childhood undernutrition. From the ancient perceptions of milk as a divine living fluid, dairy products have become agro-industrial food items providing key nutrients required for the growth and development of children. Before the availability of pasteurised milk, artificial infant feeding carried the risk of milk-borne diseases and life-threatening consequences on child health. General improvements in hygiene and technical innovations in the middle of the 19th century have facilitated the industrial production of breast milk substitutes.
The historical perspective reveals that dietary interventions were often dominated by unchallenged doctrines and many decades passed before research findings could reject some of them. This applies to the aetiological concept of alimentary toxins contributing to wasting/marasmus, while the ‘germ theory’ delayed the recognition of diseases caused by nutritional deficiencies. Similarly, the assumption that protein deficiency is the main cause of severe malnutrition contributed to an increased protein content in therapeutic feeding regimens. However, study results in the 1960s and 1970s challenged this ‘protein dogma’, the focus of research shifted to the role of energy and micronutrient deficiencies and with respect to protein from quantitative to qualitative considerations.
Regarding dietary therapies of children affected by SAM, research supports improved efficacy with inclusion of milk powder. This has led to the development of RUTF allowing out-patient management of severely undernourished children without medical complications. However, the following strategy of using imported expensive milk products to treat moderate forms of MAM is critically viewed as a supply- rather than a demand-driven approach. Studies revealed the community production of ready-to-use foods with locally available food leads to better acceptance, lower costs and offers opportunities for local employment.
Current nutritional interventions favour the inclusion of milk products due to their high density and bioavailability of nutrients such as high-quality protein and key micronutrients. Regarding negative long-term consequences of rapid catch-up weight associated with the use of cows’ milk products there is some evidence that these risks may be minimised by using whey protein products.
However, many challenges and research gaps remain in the use of milk-based products applied in the dietary management of childhood undernutrition. Comprehensive interventions are needed to address the multifactorial causes of undernutrition and the specific requirements of children of different age groups living in various settings. Finally, the high costs of commercial milk products need to be compared with equally funded community-based child care and nutrition programmes supporting and promoting breast-feeding and healthy complementary foods that are locally available.
Acknowledgements
There are no conflicts of interest.




