Obesity: epidemiology and pathophysiological description
Obesity is defined as a condition of body weight gain due to abnormal or excessive fat accumulation caused by dysregulation of energy balance( 1 ). The common parameter to classify adult adiposity is the BMI, calculated according to the following formula: body weight in kilograms divided by the square of height in metres (kg/m2). Individuals with a BMI equal to or higher than 25 are overweight (BMI ≥25 kg/m2), those with a BMI of 30 or higher are generally considered obese (BMI ≥30 kg/m2)( 1 ).
In 2014, the WHO estimated that in the general population, over 1·9 billion adults were overweight and, among these, over 600 million were obese( 1 ). The worldwide prevalence of obesity from 1980 to 2014 has been more than doubled, leading to enormous medical and social costs. Indeed, obesity is strongly linked to several medical co-morbidities, including type 2 diabetes( Reference Masoodi, Kuda and Rossmeisl 2 ), CVD( Reference Van Gaal, Mertens and De Block 3 ), gastrointestinal disorders( Reference Mushref and Srinivasan 4 ) and cognitive dysfunction( Reference Sellbom and Gunstad 5 ). The energy imbalance underlying obesity is due to excessive energy and food intake (especially high-fat diets; HFD) and insufficient physical activity, resulting from social lifestyle trend modifications. Individual metabolism and genetic predisposition can also lead to an increase in adipose tissue( Reference Costa and Duarte 6 ).
Of interest, in recent years, the role of adipose tissue in the regulation of energy metabolism has been largely investigated and appreciated. Indeed, it not only accumulates and releases lipids, but acts also as an endocrine organ with important roles in both local and systemic cellular homeostasis( Reference Coelho, Oliveira and Fernandes 7 ). During obesity, adipocytes, the main cell component of adipose tissue, grow in size and induce the release of chemokines designated also as adipokines, with a consequent macrophage infiltration( Reference Vieira-Potter 8 ). Once infiltrated into adipose tissue, macrophages enhance the circulating levels of pro-inflammatory cytokines, including TNF, IL-6 and IL-1β, which account for a condition of systemic chronic low-grade inflammation( Reference Vieira-Potter 8 ). Following inflammation, an increased uptake of O2 gives rise to the production and release of reactive species at the whole-body level, with consequent malfunction of several organs and the onset of chronic systemic pathologies( Reference Johnson, Milner and Makowski 9 ).
Oxidative stress and obesity
Oxidative stress is a process generated by an imbalance between the production of reactive oxygen species (ROS) and antioxidant defence mechanisms( Reference Duracková 10 ). In particular, it occurs when an excessive production of ROS overwhelms the body’s antioxidant defence systems or when there is a significant decrease or lack in the efficacy of antioxidant mechanisms( Reference Bjørklund and Chirumbolo 11 ).
ROS comprise all the chemical species with unpaired electrons, such as singlet oxygen and H2O2, which oxidise other molecules to gain electrons and stabilise themselves. The most common cellular free radicals include the hydroxyl radical (OH·), the superoxide anion (O2·−), H2O2, the peroxy radical (ROO−), the perhydroxyl radical (HO2∙), NO (∙NO) and peroxynitrite (ONOO−)( Reference Genestra 12 ). In mammalian cells, the main sources for the production of free radicals comprise mitochondria, peroxisomes (mainly peroxisomal oxidase), the endoplasmic reticulum (primarily cytochrome P-450 isozymes), plasma membranes (NADPH oxidase) and extracellular space (xanthine oxidase). At low concentrations, ROS act as mediators regulating an array of physiological functions, a process designated as redox signalling. By contrast, at high concentrations ROS can lead to oxidation of biological macromolecules, such as proteins, lipids, carbohydrates and nucleic acids, thus contributing to the pathogenesis of several chronic diseases( Reference Halliwell and Gutteridge 13 ). ROS can activate redox-sensitive signalling pathways, including NF-κB and mitogen-activated protein kinase, as well as transcription factors, such as signal transducer and activator of transcription 3 (STAT3), hypoxia-inducible factor-1α (HIF-1α), activator protein-1 (AP-1), nuclear factor of activated T cells (NFAT) and NF-E2-related factor-2 (Nrf2), which mediate immediate cellular stress responses, leading to a vicious circle( Reference Arulselvan, Fard and Tan 14 ) (Fig. 1). Induction of cyclo-oxygenase-2 and inducible NO synthase (iNOS) as well as alterations in the expression of specific microRNA have also been reported to play a role in pathological events associated with oxidative stress( Reference Arulselvan, Fard and Tan 14 ).

Fig. 1 Graphical presentation of oxidative stress–molecular targets. MAPK, mitogen activated protein kinase; HIF-1α, hypoxia-inducible factor-1α; Nrf2, NF-E2 related factor-2; AP-1, activator protein-1; STAT3, signal transducer and activator of transcription 3; NFAT, nuclear factor of activated T cells.
A growing body of evidence highlights the relevance of oxidative stress in obesity and related co-morbidities. Of interest, Furukawa et al. ( Reference Furukawa, Fujita and Shimabukuro 15 ) pointed out the increased oxidative stress in accumulated fat as a key pathogenic mechanism of obesity in both human subjects and rodents. Considerable evidence supports the notion that different adipose depots may differ dramatically in terms of physiological meaning and pathophysiological significance( Reference Liu, Pulliam and Liu 16 ). Indeed, excess accumulation of visceral fat in the abdominal cavity has been associated with increased risks of metabolic dysfunction, diabetes and CVD mortality( Reference Gesta, Tseng and Kahn 17 , Reference Pischon, Boeing and Hoffmann 18 ). By contrast, subcutaneous fat, primarily located in depots under the skin, appears to be relatively benign in nature( Reference Després 19 ). At present, the intimate link between the location of fat accumulation, metabolic disease risk and depot-specific differences is well established. However, it remains largely unclear how these differences between depots are regulated at the molecular level. In this regard, a recent study has identified a critical molecular regulator that mediates differential inflammatory and oxidative responses of the visceral and subcutaneous fat induced by excess nutritional stress( Reference Qiang, Kong and Fang 20 ).
Several mechanisms have been proposed to explain the enhanced oxidative stress in the presence of obesity, including disorders of mitochondrial and peroxisomal fatty acid oxidation, hyper-consumption of O2 and impairment of antioxidant defences( Reference Manna and Jain 21 ). In the adipose tissue, mitochondrial and peroxisomal oxidation of fatty acids can produce ROS in oxidative reactions, while hyperconsumption of O2 generates free radicals in the mitochondrial respiratory chain. In addition, in obese humans and rodents the levels of oxidative stress-associated markers have been found to be elevated in plasma and urine samples( Reference Furukawa, Fujita and Shimabukuro 15 ). Notably, the expression and activity of antioxidant enzymes, such as superoxide dismutase, catalase and glutathione peroxidase, have been reported to be significantly reduced in the adipose tissue of obese individuals( Reference Manna and Jain 21 ).
Blunting the excess of oxidative stress: dietary strategies
Emerging evidence, from both experimental and epidemiological studies, has shown that an antioxidant-rich diet could contribute to protection against free radical production and oxidative damage, induction of antioxidant signalling pathways, enhancement of the endogenous antioxidant defence system, attenuation of oxidative stress, with consequent prevention of obesity and related co-morbidities. Globally, there is an upsurge of interest in the therapeutic potential of medicinal plants, the beneficial effect of which has been ascribed mainly to phenolic molecules, with particular regard for flavonoid compounds( Reference Kawser Hossain, Abdal Dayem and Han 22 ). Dietary flavonoids might be considered as anti-obesity agents, since they can reduce adipose tissue mass, thereby decreasing intracellular free radical formation( Reference Kawser Hossain, Abdal Dayem and Han 22 ). In the following sections, the most important antioxidants flavonoid compounds, commonly employed as dietary supplements in the management of diet-induced obesity, and their outcomes are discussed. A summary of major findings obtained with flavonoids in counteracting obesity and related disorders in experimental models and in epidemiological/clinical studies is provided in Tables 1 and 2, respectively.
Table 1 Summary of the effects of flavonoids in counteracting obesity and related disorders in experimental models

HFD, high-fat diet; NAFLD, non-alcoholic fatty liver disease; Nrf2, NF-E2-related factor-2; MDA, malondialdehyde; iNOS, inducible NO synthase; ICAM-1, intercellular adhesion molecule 1; SOD, superoxide dismutase; RBP4, retinol-binding protein-4; MCP-1, monocyte chemoattractant protein-1; FoxO1, forkhead box protein O1; SREBP-1c, sterol regulatory element-binding protein 1c; GPX, glutathione peroxidase α1 phosphorylation; HC, high carbohydrate; AMPK, adenosine monophosphate-activated protein kinase; SIRT1, silent information regulator 1.
Table 2 Summary of the effects of flavonoids in counteracting obesity and related disorders in epidemiological and clinical studies

Flavonoid compounds
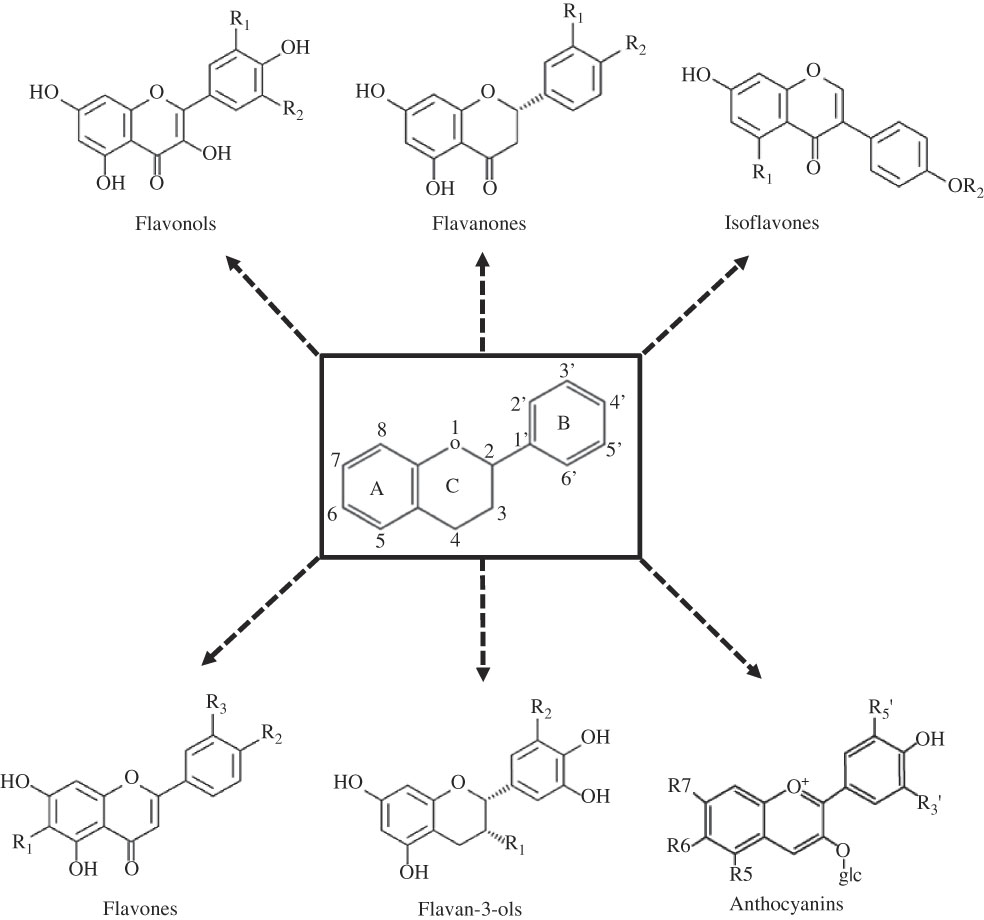
Flavonoids comprise a large family of about 8000 different hydroxylated polyphenolic compounds, which are extensively distributed in beverages, vegetables and fruits( Reference Kawser Hossain, Abdal Dayem and Han 22 ). Structurally, flavonoids are based upon a fifteen-carbon skeleton consisting of two benzene rings (A and B) linked via a heterocyclic pyrane ring (C)( Reference Havsteen 23 ). Flavonoids differ in the level of oxidation and pattern of substitution of the C ring, whereas individual compounds within a class differ in the substitution pattern of the A and B rings( Reference Havsteen 23 ) (Fig. 2).

Fig. 2 Basic chemical structures of flavonoids and their classification. glc, Glucose.
Flavonoids are classified into flavonols (i.e. quercetin, kaempferol, resveratrol and myricetin), flavanones (i.e. eriodictyol, hesperetin, naringenin and naringin), isoflavonoids (i.e. daidzein, genistein and glycitein), flavones (i.e. apigenin and luteolin), flavan-3-ols (i.e. catechins and epigallocatechin gallate) and anthocyanins (cyanidin)( Reference Kawser Hossain, Abdal Dayem and Han 22 ) (Fig. 2). Flavonols are the subgroup of flavonoids most widely contained in vegetables, such as onions, leeks, Brussels sprouts, kale, broccoli, tea, berries, beans and apples. Flavonone compounds are found abundantly in citrus fruits. Isoflavones are highly concentrated in soyabeans and soya products, as well as legumes. Good sources of flavones include celery, parsley, various herbs and hot peppers. Flavanols are found in green and white teas, cocoa, grapes, apples, berries, fava beans and red wine. Anthocyanidins are abundant in red, purple and blue berries, pomegranates, plums, red wine as well as red and purple grapes( Reference Havsteen 23 ).
Flavonoids are of particular interest, owing to their preventive role against several human diseases, arising from their wide spectrum of biological actions, including anti-inflammatory, antioxidant, antiviral, anticancer and neuroprotective activities. Nowadays, major attention is being focused on the free radical-scavenging and metal ion-chelating activity of these compounds( Reference Kawser Hossain, Abdal Dayem and Han 22 ). Of note, flavonoids have the ability of inhibiting the enzymes responsible for superoxide production (i.e. xanthine oxidase and protein kinase C) as well as cyclo-oxygenase, lipoxygenase, microsomal succinoxidase, NADH oxidase, and to exert also an inhibitory effect on the expression of iNOS, largely related to oxidative stress conditions( Reference Kawser Hossain, Abdal Dayem and Han 22 ).
Flavonoids, oxidative stress and obesity
Over the last decade, there has been an interest in the expansion of anti-obesity drugs, with particular attention paid to those from natural sources. Extensive investigations have been conducted to identify dietary components that may influence the accumulation of excess body fat. Among such components, flavonoid compounds hold a key role( Reference Zhang, Gan and Li 24 ). Accordingly, in vitro and in vivo studies have suggested the anti-obesity activity of a number of flavonoids isolated from fruit and plant extracts. Among them, genistein has been reported to be a promising therapeutic agent to regulate obesity-related inflammation and oxidative stress in pancreatic β-cells( Reference Behloul and Wu 25 ). Dietary supplementation of 0·1 % quercetin in the diet for 12 weeks has been found to reduce body weight gain, improve insulin sensitivity and glucose intolerance and suppress adipose tissue macrophage infiltration and inflammation through the modulation of adenosine monophosphate-activated protein kinase (AMPK) α1 phosphorylation and silent information regulator 1 (SIRT1) pathway in mice receiving a HFD( Reference Dong, Zhang and Zhang 26 ). Dietary kaempferol (0·15 % of diet for 92 d) reduced the accumulation of adipose tissue by increasing lipid metabolism, through the down-regulation of PPAR-γ and sterol regulatory element-binding protein 1c (SREBP-1c), and increased the antioxidant defences in obese mice( Reference Zang, Zhang and Igarashi 27 ). Administration of myricetin at 150 mg/kg per d for 2 weeks significantly reduced body weight, serum glucose, TAG and cholesterol in mice with diet-induced obesity. Moreover, in the same study, obesity-associated oxidative stress (glutathione peroxidase activity, total antioxidant capacity and malondialdehyde) and inflammation (TNF) were also ameliorated( Reference Su, Feng and Zheng 28 ). Apigenin has been found to be effective in restraining non-alcoholic fatty liver disease progression as well as in attenuating lipid accumulation and oxidative stress via Nrf2 activation in HFD-fed mice( Reference Feng, Yu and Li 29 ). In a recent study, flavonoid-rich extract (apigenin, luteolin, rutin, luteolin-7-O-glucoside, kaempferol-3-O-glucoside, apigenin-7-O-glucoside and quercetin-3-O-glucoside) from Paulownia fortunei flowers (EPF) at doses of 50 or 100 mg/kg for 8 weeks was found to have potent protective effects against hyperlipidaemia, hepatic lipid accumulation and insulin resistance in HFD-fed mice and human HepG2 hepatocytes cells. The protective effects of EPF have been associated, at least in part, with decreased lipogenesis, increased glucose metabolism and induced fatty acid oxidation in the liver by the AMPK pathway, a key regulator of energy metabolic homeostasis( Reference Liu, Ma and Sun 30 ). In thirty-two adult obese patients with BMI from 30 to 40 kg/m2, resveratrol (150 mg/d) and catechin-rich grape seed extract (400 mg/d) intake for 28 d exerted an antioxidant activity, reducing the expression of 200 redox-related genes( Reference De Groote, Van Belleghem and Devière 31 ). In a recent pilot study in overweight/obese women, the consumption of an orange juice rich in anthocyanins at a dose of 500 ml daily over a period of 12 weeks improved LDL-cholesterol( Reference Azzini, Venneria and Ciarapica 32 ).
Prevention of obesity-related co-morbidities: a flavonoid-based nutraceutical approach
Diabetes type 2 mellitus
Type 2 diabetes mellitus, the most common type of diabetes, is a long-term metabolic disorder characterised by impairments of both insulin secretion and action( Reference Hardy, Czech and Corvera 33 ). In obese individuals, the high levels of circulating pro-inflammatory cytokines contribute actively to the induction of insulin resistance( Reference Nowotny, Jung and Höhn 34 ). Insulin resistance generates compensatory hyperisulinaemia with overstimulation of pancreatic β-cell function and induction of insulin receptors( Reference Nowotny, Jung and Höhn 34 ). Furukawa et al. have reported that the increased oxidative stress in accumulated visceral fat is, at least in part, the cause of dysregulated adipocytokine secretion and the metabolic syndrome underlying diabetes( Reference Furukawa, Fujita and Shimabukuro 15 ). Indeed, in cultured adipocytes elevated levels of fatty acids increase oxidative stress, via NADPH oxidase activation, that in turn causes the dysregulated production of adipocytokines( Reference Furukawa, Fujita and Shimabukuro 15 ). In addition, the authors reported that the treatment with inhibitors of NADPH oxidase reduced ROS production, attenuated adipocytokine production and improved diabetes in obese mice( Reference Furukawa, Fujita and Shimabukuro 15 ).
Flavonoids are being actively studied as potential regulatory compounds of signalling pathways related to the development of diabetes. Cyanidin-3-O-β-glucoside (50 µmol/l) and its metabolite protocatechuic acid (100 µmol/l) counteracted completely the impairment of the glucose transport mechanism induced by circulating oxidised LDL, exerting insulin-like effects in human omental and murine adipocytes( Reference Scazzocchio, Vari and Filesi 35 ). Moreover, the authors observed that the increase in glucose uptake by adipocytes was mediated by an enhanced PPARγ activity, which promotes fatty acid storage in fat depots and regulates the expression of adipocyte-secreted hormones that influence glucose homeostasis( Reference Scazzocchio, Vari and Filesi 35 ). Along the same line, another study reported that dietary cyanidin-3-O-β-glucoside (0·2 % of diet for 5 weeks) ameliorated hyperglycaemia and enhanced insulin sensitivity in diabetic mice( Reference Sasaki, Nishimura and Hoshino 36 ). In particular, cyanidin-3-O-β-glucoside up-regulated Glut4 and down-regulated adipokine retinol-binding protein-4 in white adipose tissue, these effects being followed by down-regulation of the inflammatory adipocytokines (monocyte chemoattractant protein-1 (MCP-1) and TNF)( Reference Sasaki, Nishimura and Hoshino 36 ). Genistein has been shown to regulate the adipocyte life cycle, to lower diabetes inflammation and to exert oxidative stress, and have protective effects on pancreatic β-cells( Reference Behloul and Wu 25 ). Both apigenin and naringenin, intragastrically administered at a dose of 50 or 100 mg/kg once per d for 6 weeks, significantly decreased the levels of blood glucose, serum lipids, malondialdehyde and intercellular adhesion molecule 1 (ICAM-1), increased superoxide dismutase activity, and improved NO production in diabetic rats( Reference Ren, Qin and Wu 37 ). Furthermore, in another study, a novel orange peel hydroethanolic extract, composed of naringin and naringenin compounds at a dose of 100 mg/kg body weight per d for 4 weeks, exerted a potent anti-diabetic effect in diabetic rats via their anti-hyperglycaemic and anti-hyperlipidaemic properties as well as antioxidant activities( Reference Ahmed, Hassan and Abdel-Twab 38 ). A study by Alam et al. ( Reference Alam, Meerza and Naseem 39 ) described an antioxidant potential and protective effect of quercetin associated with a significant increase in glutathione, superoxide dismutase, catalase and glutathione-S-transferase levels in diabetic mice. Guo et al. ( Reference Guo, Xia and Zou 40 ) reported that dietary cyanidin-3-glucoside supplementation (0·2 %) for 5 weeks significantly lowered fasting glucose levels, markedly improved insulin sensitivity and alleviated hepatic TAG content and steatosis in both HFD-fed and genetically diabetic db/db mice. In addition, this flavonoid compound also reduced RNA levels and serum concentrations of inflammatory cytokines (TNF, MCP-1 and IL-6) as well as macrophage infiltration in adipose tissue by modulating the transcription factor forkhead box protein O1 (FoxO1)( Reference Guo, Xia and Zou 40 ). In recent epidemiological studies, Grosso et al. ( Reference Grosso, Stepaniak and Micek 41 , Reference Grosso, Stepaniak and Micek 42 ) reported that individuals with higher total dietary flavonoid intake were associated with lower risk of both impaired glucose metabolism( Reference Grosso, Stepaniak and Micek 41 ) and diabetes( Reference Grosso, Stepaniak and Micek 42 ). In addition, findings from a meta-analysis of prospective cohort studies on the potential benefits of dietary flavonoids intake on diabetes supported the evidence of a positive correlation between total flavonoid consumption and decreased risk of type 2 diabetes( Reference Liu, Zhan and Liu 43 ). Moreover, a meta-analysis of randomised controlled trials showed that the administration of green tea catechins in adults resulted in a significant reduction of fasting blood glucose( Reference Zheng, Xu and Li 44 ).
CVD
Obesity has long been known to be associated with an increased development of CVD( Reference Van Gaal, Mertens and De Block 3 ). Both increased BMI and waist circumference (abdominal fat marker) represent two important cardiovascular risk factors. Mortality and morbidity associated with CVD have been found to be elevated in overweight individuals, particularly in the presence of visceral deposition of adipose tissue( Reference Van Gaal, Mertens and De Block 3 ). Obesity may be associated with hypertension, dyslipidaemia, diabetes and elevated levels of fibrinogen and C-reactive protein, all being related to an increase in the risk of CVD onset. Accumulating evidence suggests that dysfunctional innate and adaptive immune and inflammatory responses, along with the overproduction of oxidants, contribute to the pathogenesis of vascular dysfunction and hypertension in obese patients( Reference Van Gaal, Mertens and De Block 3 ). An increased oxidative stress underlies the pathophysiology of hypertension and atherosclerosis by directly affecting vascular wall cells. Indeed, high ROS production along with a decrease in antioxidant capacity lead to endothelial dysfunction, characterised by a reduced bioavailability of vasodilators, particularly NO, and an increase in endothelium-derived contractile factors, favouring atherosclerotic disease( Reference Reho and Rahmouni 45 ).
Preclinical studies( Reference Xia, Ling and Zhu 46 – Reference Gandhi, Upaganalawar and Balaraman 49 ) have observed that the intake of flavonoid compounds (i.e. anthocyanin, quercetin, apigenin and hesperidin) reduced the risk of CVD by decreasing oxidative stress and related inflammation. In particular, anthocyanin protected against CD40-induced proinflammatory signalling in endothelial cells by regulating cholesterol distribution( Reference Xia, Ling and Zhu 46 ). Quercetin attenuated inflammation in human umbilical vein endothelial cells (HUVEC) through the reduction of both cytokines and MCP-1 expression, all involved in monocyte recruitment during the early stages of atherosclerosis( Reference Tribolo, Lodi and Connor 47 ). Apigenin inhibited high glucose and TNF-induced adhesion molecule expression such as expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in human endothelial cells, therefore reducing the development of atherosclerotic vascular diseases( Reference Yamagata, Miyashita and Matsufuji 48 ). Hesperidin protected against focal myocardial ischaemia/reperfusion injury-induced arrhythmias in rats by promoting a significant increase in tissue nitrite, antioxidant levels and reduction of inflammation, arrhythmias and apoptosis( Reference Gandhi, Upaganalawar and Balaraman 49 ). In an experimental model of cardiac hypertrophy induced by ROS, changes in Ca transport can further lead to tissue injury, cell killing and impaired cardiac contraction. In this setting, some flavonoids significantly increased cell viability and protected against cardiomyocyte apoptosis, via preservation of sarco/endoplasmic reticulum Ca ATPase (SERCA) function, which might represent a novel strategy against CVD associated with oxidative stress( Reference Horáková 50 ).
Naringin has been found to normalise systolic blood pressure and improve vascular and cardiac dysfunction via reduction of inflammatory cell infiltration, decreasing oxidative stress, lowering plasma lipid concentrations, and improving liver mitochondrial function in high-carbohydrate and HFD-fed rats( Reference Alam, Kauter and Brown 51 ). Wild blueberries, an important source of dietary anthocyanins, reduced the development of systemic inflammation and prevented the progression of chronic hypertension in HFD-fed mice, thus supporting their potential role in counteracting the adverse health effects associated with obesity development( Reference Mykkänen, Huotari and Herzig 52 ). Furthermore, genistein prevented hyperglycaemia-induced monocyte adhesion to human aortic endothelial cells through preservation of the cAMP signalling pathway, and ameliorated vascular inflammation in obese diabetic mice( Reference Babu, Si and Fu 53 ).
In a clinical study, Clerici et al. ( Reference Clerici, Setchell and Battezzati 54 ) reported that isoflavone-enriched soya germ pasta (80 g serving/d) reduced serum lipids and improved cardiovascular risk markers such as arterial stiffness and high-sensitivity C-reactive protein in hypercholesterolaemic adults. Epidemiological evidence suggested that fruits containing relatively high concentrations of flavonols, anthocyanins and procyanindins, such as pomegranate, purple grapes and berries, were effective at reducing CVD risk factors, particularly with respect to anti-hypertensive effects, inhibition of platelet aggregation and increasing endothelial-dependent vasodilation( Reference Chong, Macdonald and Lovegrove 55 ). A recent epidemiological study, conducted on adults in the USA, suggested that a high flavonoid intake (mainly anthocyanidins, flavones and flavanones) is inversely correlated with the risk of CVD, as assessed by BMI and waist circumference( Reference Sebastian, Wilkinson Enns and Goldman 56 ). Of note, the intake of flavonoids tested in this study was based on flavonoid-rich foods and beverages, but not supplements, reported in What We Eat in America (WWEIA), the dietary intake component of the National Health and Nutrition Examination Survey (NHANES) 2007–2010( Reference Sebastian, Wilkinson Enns and Goldman 56 ).
Gastrointestinal dysfunctions
Obesity is linked to gastrointestinal disorders, that can occur as gastro-oesophageal reflux disease, dyspepsia, constipation, irritable bowel syndrome, diarrhoea, bloating and other non-specific conditions( Reference Mushref and Srinivasan 4 , Reference Ho and Spiegel 57 , Reference Buchoucha, Fysekidis and Julia 58 ).
Preclinical studies, aimed at characterising the molecular mechanisms underlying gastrointestinal disturbances in obesity, reported that diet-induced obesity determines a remarkable morpho-functional remodelling of the enteric neuromuscular compartment( Reference Bhattarai, Fried and Gulbransen 59 , Reference Antonioli, Pellegrini and Fornai 60 ), followed by alterations in gut transit( Reference Chong, Macdonald and Lovegrove 55 ). Several lines of evidence indicate the presence of an increased mucosal permeability and changes in the intestinal microbiota, along with low-grade enteric inflammation and oxidative stress in the bowel tissues of obese animals( Reference Anitha, Reichardt and Tabatabavakili 61 – Reference Stenman, Holma and Korpela 64 ), leading to hypothesise a critical role of this phlogistic condition in the pathophysiology of intestinal dysfunctions associated with obesity( Reference Savini, Catani and Evangelista 65 ). Although several studies have implicated the adipose tissue as being primarily responsible for obesity-associated inflammation, the most recent findings have correlated the impairments of intestinal immune homeostasis and mucosal barrier with an increased activation of inflammatory pathways and the development of insulin resistance( Reference Savini, Catani and Evangelista 65 ). On this basis, it is now essential to characterise the mechanisms underlying obesity-associated gut alterations for developing novel therapeutic approaches to prevent obesity and its associated diseases.
At present, flavonoid compounds appear to be promising therapeutic candidates, among the variety of natural treatments that have been identified to date. Indeed, there is growing evidence that flavonoids could exert a protective role in diet-induced obesity by modulation of intestinal inflammation, barrier integrity and gut microbiota composition( Reference Gil-Cardoso, Ginés and Pinent 66 ). Dietary (–)-epigallocatechin-3-O-gallate contributes to the beneficial effects of green tea on diabetes, obesity and cancer by modulating the gene expression of gluconeogenesis (in particular, human hepatocyte nuclear factor 1α (HNF1α), HNF4, glucose-6-phosphate and phosphoenolpyruvate carboxykinase) in the mouse intestine( Reference Yasui, Tanabe and Miyoshi 67 ). Treatment with cranberry extract, containing flavonols, anthocyanins and proanthocyanidins, has been found to reduce high-fat/high-sucrose-induced weight gain, visceral obesity and to alleviate intestinal inflammation and oxidative stress in C57BL/6J mice( Reference Anhê, Roy and Pilon 68 ). Grape polyphenols, abundant in anthocyanins, have be found to act in the intestine and modify the gut microbial community structure, resulting in a reduction of intestinal and systemic inflammation, and improving metabolic outcomes( Reference Roopchand, Carmody and Kuhn 69 ). In particular, grape polyphenols attenuated weight gain, adiposity, serum inflammatory markers (TNF, IL-6 and lipopolysaccharide) and glucose intolerance in HFD-fed mice. Moreover, grape polyphenols lowered the intestinal expression of inflammatory and oxidative stress markers (i.e. TNF, IL-6 and iNOS), increased the intestinal expression of proteins involved in barrier function (occludin) and limited TAG storage( Reference Roopchand, Carmody and Kuhn 69 ). In HFD-fed mice, apigenin counteracted systemic metabolic alterations and enteric inflammation, and normalised colonic dysmotility associated with obesity. In particular, apigenin reduced malondialdehyde, IL-1β and IL-6 colonic levels, as well as substance P and iNOS expression with a normalisation of colonic electrically evoked tachykininergic and nitrergic contractions( Reference Gentile, Fornai and Colucci 70 ).
Cognitive dysfunctions
Obesity is associated with cognition decline across the lifespan( Reference Wang, Chan and Ren 71 ). Epidemic and experimental studies have confirmed that obesity can lead to neuroinflammation, neurodegenerative diseases and adversely affect cognition, such as complex attention, verbal and visual memory, and decision making in obese adults( Reference Benito-León, Mitchell and Hernández-Gallego 72 ). It has been showed that obesity induced by a HFD in mice not only caused neuronal insulin resistance, but also can induce brain mitochondrial dysfunction as well as learning impairment( Reference Wang, Yan and Chen 73 ).
Over the last decade, prospective data have suggested that high fruit and vegetable intake can improve cognitive functions and reduce the risk of neurodegenerative process development( Reference Orhan, Daglia and Nabavi 74 ). The protective effects against neurodegeneration could be in part due to the intake of flavonoids that have been associated with several health benefits, such as antioxidant and anti-inflammatory activities, increased neuronal signalling and improved metabolic functions( Reference Orhan, Daglia and Nabavi 74 ). Green tea catechin had a protective effect on brain and pancreas functions in HFD-fed mice( Reference Unno, Yamamoto and Maeda 75 ). Naringin improved neuronal insulin signalling and decreased mitochondrial dysfunction through the activation of AMPK in HFD-induced obese mice( Reference Wang, Yan and Chen 73 ). Luteolin, a common flavone found in plants, has been shown to alleviate neuroinflammation (as indicated by a decrease in TNF and IL-6 in the hippocampus), oxidative stress and neuronal insulin resistance in the brain of HFD-fed mice. In this study, luteolin protected also obese mice against HFD-induced cognitive deficiencies (i.e. performance in Morris water maze and step-through passive avoidance response). These results highlight a previously unrecognised potential of luteolin in alleviating obesity-induced cognitive impairment( Reference Liu, Fu and Lan 76 ). Other studies have shown the cognitive benefits of flavonoid supplementation (i.e. apigenin, kaempferol, luteolin, myricetin and quercetin) in improving cognitive function (i.e. learning and memory) and reducing cognitive decline( Reference Johnson 77 ). Although deserving further investigations, it has been suggested that flavonoids might exert their activities on cognition via mechanisms that include anti-inflammatory effects (i.e. decreasing neuroinflammation through targeting proinflammatory molecules such as NF-κB), scavenging of ROS and reactive nitrogen species (i.e. decreasing oxidative stress), as well as reducing brain microglial cell activation (leading to a decrease in neuroinflammation)( Reference Johnson 77 ). Of note, other studies have also shown that catechins may prevent the formation of amyloid-β plaques and enhance cognitive functions, with a consequent potential usefulness in the treatment of Alzheimer’s disease or dementia( Reference Fernando, Somaratne and Goozee 78 ). Furthermore, other phytochemicals contained in tea can exert important antioxidant properties, along with other properties capable of modulating intracellular neuronal signal transduction pathways and mitochondrial function( Reference Fernando, Somaratne and Goozee 78 ).
Concluding remarks
The prevalence of overweight and obesity and their associated co-morbidities are regarded as a major threat to public health. Although the therapeutic management of obese patients includes modifications in their lifestyles, adequate diet and exercise programmes, a proper control of weight is often low and disappointing.
While the aetiology of obesity is multi-faceted, oxidative stress and related inflammatory conditions represent potential and useful targets. In this view, great interest has been paid to investigate natural bioactive compounds, found in plants, for their antioxidant and anti-inflammatory activities in many human diseases. The strongest conclusion that can be drawn from the revision of current literature is that some flavonoids offer novel therapeutic approaches to prevent obesity and related co-morbidities through their capacity of reducing adipose tissue mass, thereby decreasing intracellular free radical formation, increasing antioxidant defences and attenuating inflammatory signalling pathways, mostly from studies in animal models. To date, although continuous efforts are being made in this research area, additional studies are still required to better elucidate the value of dietary flavonoids in the context of public health or clinical practice. In particular, further efforts should allow better understanding of the fundamental aspects of flavonoid pharmacology/toxicology, in order to issue reliable public health recommendations on the effective dose and putative adverse effects of these compounds. To promote the development of nutritional flavonoids, it appears essential also to verify whether preclinical findings could be translated into the clinical setting, with particular regards for doses and recommendations. In addition, a better elucidation of the molecular mechanisms underlying the beneficial effects of flavonoids, by means of in vitro and in vivo experiments, would provide insights into the field of drug development for the management of obesity. In conclusion, future multi-disciplinary approaches, involving epidemiological and clinical investigations, are required to further characterise the potential beneficial effects of both plant-isolated flavonoids and flavonoid-rich foods in counteracting obesity and related disorders.
Acknowledgements
The present review was financially supported by PRA_2016_20, granted by the University of Pisa.
D. G., L. A., C. B., M. F., R. C. and C. P. planned the manuscript; D. G., L. A. and C. B. wrote the manuscript. All authors read and approved the final manuscript.
The authors have no conflicts of interest to declare.