Introduction
The global diabetic population is rapidly growing from 382 million in 2013 to an estimated 592 million by 2035( Reference Guariguata, Whiting and Hambleton 1 ). This situation imposes a great socio-economic burden on public health( Reference Sherwin, Anderson and Buse 2 ). Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder of abnormal glucose and lipid metabolism, resulting in CVD, retinopathy, nephropathy, neuropathy, leg ulcers and gangrene( Reference Nathan 3 ). The risk factors for T2DM include obesity, age, genetics, smoking, sedentary lifestyle and hypertension( Reference Sherwin, Anderson and Buse 2 ). Recently, it has been proposed that changes in gut microbiota composition resulting from obesity could contribute to the pathogenesis of T2DM( Reference Backhed, Ding and Wang 4 – Reference Ley, Turnbaugh and Klein 8 ).
Probiotics and prebiotics may exert anti-diabetic effects through changes in microbiota( Reference Yadav, Lee and Lloyd 9 – Reference Tajabadi-Ebrahimi, Sharifi and Farrokhian 13 ). Beneficial modification of the gut flora by probiotic and/or prebiotic treatment could be one dietary therapy for the prevention and treatment of T2DM.
PubMed, EMBASE, Cochrane and Scopus online database were searched for human intervention studies using the following terms: probiotic OR fermented OR yogurt OR cheese OR prebiotic OR inulin OR fructo-oligosaccharide OR synbiotic OR resistant starch OR gut microbiota, PLUS glucose OR glycemic OR hyperglycemia OR insulin OR insulin sensitivity OR type 2 diabetes Plus Human trial. Reviews were also utilised to clarify the potential mechanisms by which probiotics, prebiotics and synbiotics may alter insulin sensitivity.
Gut microbiota in individuals with type 2 diabetes mellitus and obesity
Most gut micro-organisms inhabit the large intestine that contains an estimated 1011−12 bacteria per g( Reference Moreno-Indias, Cardona and Tinahones 14 ). Gut microbiota can influence host adiposity and regulate fat storage( Reference Backhed, Ding and Wang 4 , Reference Ley, Backhed and Turnbaugh 7 , Reference Jumpertz, Le and Turnbaugh 15 – Reference Shavakhi, Minakari and Firouzian 17 ).
The Bacteroidetes and the Firmicutes are groups of bacteria dominant in the human gut( Reference Ley, Turnbaugh and Klein 8 ). A correlation between changes in gut microbiota composition and obesity was reported in obese human subjects( Reference Ley, Turnbaugh and Klein 8 , Reference Turnbaugh, Hamady and Yatsunenko 18 ) and ob/ob mice( Reference Turnbaugh, Ley and Mahowald 19 ), with lower microbial diversity, increased Firmicutes and decreased Bacteroidetes, and this obesity-associated gut microbiota had an increased capacity for energy harvest from the diet( Reference Turnbaugh, Ley and Mahowald 19 ). Germ-free wild-type C57BL/6J mice colonised with caecal microbiota from obese donors showed a significant increase (47 (sd 8·3) %) in body fat, compared with 27 (sd 3·6) % in mice colonised with a microbiota from lean donors over 2 weeks. During the 2 weeks, obese microbiota recipients consumed 55·4 (sd 2·5) g chow and gained 1·3 (sd 0·2) g fat, while the lean microbiota recipients consumed 54·0 (sd 1·2) g chow and gained 0·86 (sd 0·1) g fat, a difference of 2 % of total energy consumed( Reference Turnbaugh, Ley and Mahowald 19 ).
Furthermore, composition of the intestinal microbiota in adults with T2DM was different from that in non-diabetic adults. The proportion of Firmicutes, Clostridia and bifidobacteria was significantly lower in diabetic adults than in non-diabetic adults( Reference Larsen, Vogensen and van den Berg 20 , Reference Wu, Ma and Han 21 ).
Mucin-degrading bacteria such as Akkermansia muciniphila and Desulfovibrio were enriched in samples derived from T2DM patients( Reference Qin, Li and Cai 22 ). In contrast, several recent studies showed that in mice, direct administration of Akkermansia muciniphila ( Reference Everard, Belzer and Geurts 23 – Reference Schneeberger, Everard and Gomez-Valades 27 ) or specific proteins isolated from the outer of membrane of Akkermansia muciniphila could prevent obesity, insulin resistance as well as atherosclerosis and a human study also showed that the abundance of Akkermansia muciniphila was associated with glucose homeostasis and body fat composition( Reference Dao, Everard and Aron-Wisnewsky 28 ).
Vrieze et al. ( Reference Vrieze, Van Nood and Holleman 29 ) investigated the effect of altering the gut microbiota on insulin sensitivity in subjects with the metabolic syndrome. Obese subjects given a small-intestinal infusion of faeces from lean donors (n 9) have shown improved peripheral insulin sensitivity after 6 weeks (median rate of glucose disappearance changed from 26·2 to 45·3 µmol/kgpermin; P<0·05), as assessed by the two-step hyperinsulinaemic–euglycaemic clamp method. These subjects also have shown a 2·5-fold increase in butyrate-producing gut microbiota, Roseburia intestinalis, compared with obese subjects reinfused with their own faeces (n 9). The faecal microbiota of obese subjects had lower microbial diversity, and contained higher amounts of Bacteroidetes and lower amounts of Clostridium cluster XIVa compared with faecal microbiota after lean donor infusion at 6 weeks( Reference Vrieze, Van Nood and Holleman 29 ).
Germ-free mice which are protected from diet-induced obesity had increased phosphorylated 5'-AMP-activated protein kinase (AMPK) in skeletal muscle and liver and increased fatty acid oxidation enzymes (acetyl CoA carboxylase; carnitine palmitoyltransferase), while germ-free knockout mice deficient in fasting-induced adipose factor (FIAF), a circulating lipoprotein lipase inhibitor, were not resistant to diet-induced obesity. Germ-free FIAF-deficient animals fed a Western diet showed decreased expression of the peroxisomal proliferator activated receptor-γ coactivator 1α (PGC1α) which is known to increase genes encoding regulators of mitochondrial fatty acid oxidation in the gastrocnemius muscle compared with germ-free FIAF+/+ littermates, while there were no differences in phosphorylated AMPK levels between both groups. Consequently, germ-free mice were protected from diet-induced obesity through increased FIAF by inducing PGClα and also through elevated AMPK activity, implicating that obese gut microbiota can be responsible for decreased fatty acid oxidation and decreased FIAF/AMPK within the adipose tissue and liver( Reference Bäckhed, Manchester and Semenkovich 30 ).
The lipopolysaccharide (LPS) endotoxins, found in the outer membrane of some species of Gram-negative bacteria (for example, Neisseria spp. and Haemophilus spp.), induce signalling by binding to Toll-like receptor-4 present on endothelial cells, macrophages and monocytes. This promotes pro-inflammatory cytokines, chemokines, adhesion molecules and reactive oxygen species. An increase in LPS has been directly associated with insulin resistance( Reference Cani, Amar and Iglesias 31 ). Cani et al. ( Reference Cani, Bibiloni and Knauf 32 ) found in a mouse model that high-fat feeding changes gut microbiota with a marked reduction in some bacteria (Lactobacillus spp. and Bacteroides–Prevotella spp.), leading to an increased intestinal permeability, and LPS absorption. This increased metabolic endotoxaemia initiates adipose tissue inflammation (plasminogen activator inhibitor-1 and IL-1 mRNA), macrophage infiltration markers (macrophage chemoattractant protein-1 (MCP-1) mRNA, F4/80 mRNA) and oxidative stress (NADPHox mRNA, visceral adipose tissue and six transmembrane protein of prostate 2 (STAMP2, known to regulate nutrient-derived and inflammatory signals coordinately for metabolic homeostasis( Reference Wellen, Fucho and Gregor 33 , Reference Waki and Tontonoz 34 ))).
Even though intestinal microbiota may play a role in the aetiology of obesity and insulin resistance, the relationship between the bacteria and these metabolic disorders remains a matter of debate and most publications merely report associations between intestinal microbial composition and metabolic disorders such as obesity and T2DM( Reference Bouter, van Raalte and Groen 35 ).
Probiotics and effects of probiotics on glucose metabolism in human interventions
According to the FAO/WHO, probiotics are ‘live microorganisms which, when administered in adequate amounts, confer a health benefit on the host’( Reference Araya, Morelli and Reid 36 ). The requirement of adequate amounts differs between countries: products must contain at least 107 colony-forming units (CFU)/g of probiotic bacteria in Japan, at least 108 CFU/g probiotic bacteria in USA and 109 CFU/g probiotic bacteria in Canada. In general, >106–108 CFU/g, or >108–1010 CFU/d of viable cells are regarded efficacious( Reference Homayoni Rad, Mehrabany and Alipoor 37 , Reference Champagne, Ross and Saarela 38 ), but the cell count levels recognised do not guarantee a health effect( Reference Champagne, Ross and Saarela 38 , Reference Reid 39 ). Moreover, it is suggested that the recommendation for CFU determination should be established using accurate and frequent assessments because the number of viable cells is reduced during production, processing and formulation( Reference Champagne, Ross and Saarela 38 ).
Some of the species are: (1) lactic acid-producing bacteria (Lactobacillus, Bifidobacterium, Streptococcus); (2) non-lactic acid-producing bacterial species (Bacillus, Propionibacterium); (3) non-pathogenic yeasts (Saccharomyces; for example, Saccharomyces boulfecesardii, a non-colonising lactic acid-producing yeast); (4) non-spore-forming and non-flagellated rod or coccobacilli( Reference Saraf, Shashikanth and Priy 40 ). Over 100 Lactobacillus and over thirty Bifidobacterium species have been identified( Reference Saraf, Shashikanth and Priy 40 ). Lactic-producing Bifidobacterium and Lactobacillus are the predominant and subdominant probiotic groups( Reference Bermudez-Brito, Plaza-Diaz and Munoz-Quezada 41 ).
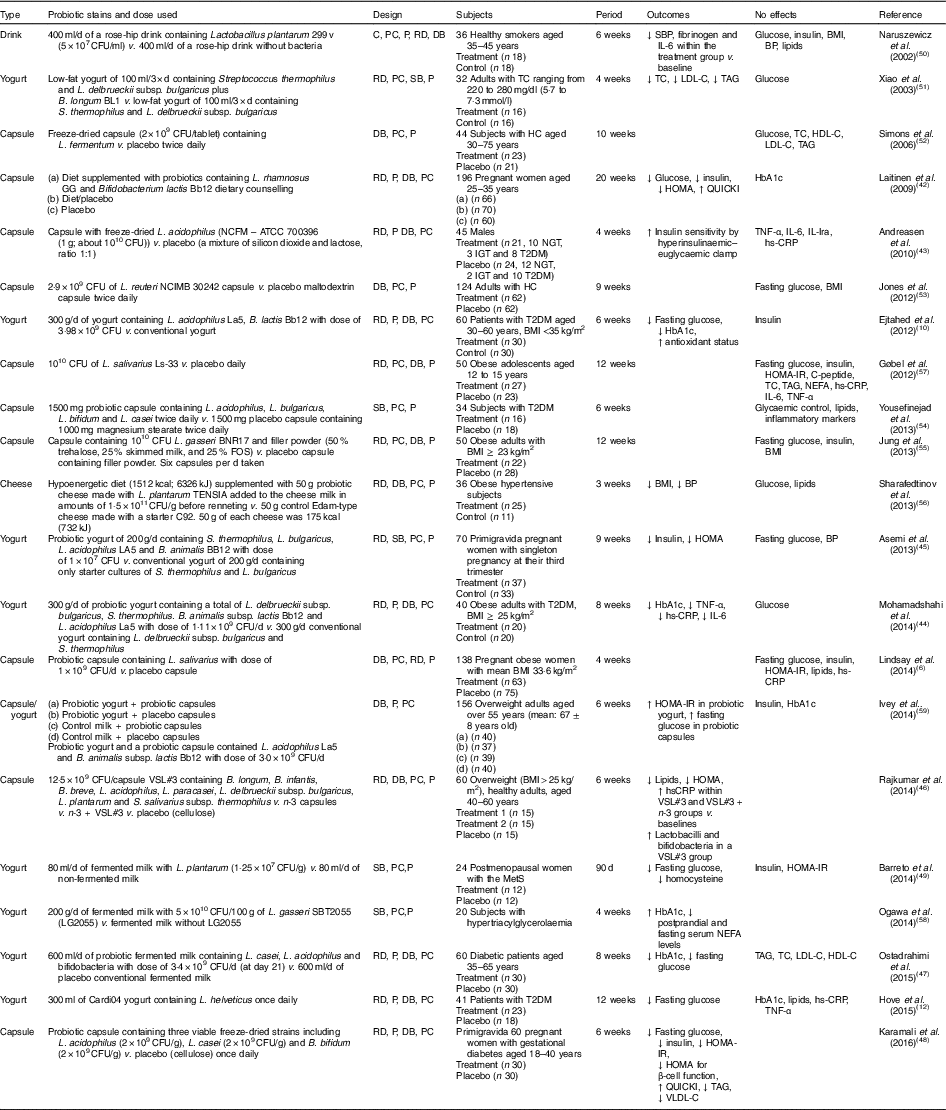
Human interventions with probiotics are shown in Table 1. Ten interventions( Reference Ejtahed, Mohtadi-Nia and Homayouni-Rad 10 , Reference Hove, Brons and Faerch 12 , Reference Laitinen, Poussa and Isolauri 42 – Reference Barreto, Colado Simao and Morimoto 49 ) have shown positive effects of probiotics on glucose control. Ten interventions( Reference Lindsay, Kennelly and Culliton 6 , Reference Naruszewicz, Johansson and Zapolska-Downar 50 – Reference Ogawa, Kadooka and Kato 58 ) have shown no effect and two interventions have shown negative effects( Reference Ivey, Hodgson and Kerr 59 ).
Table 1 Summary of probiotic human intervention studies
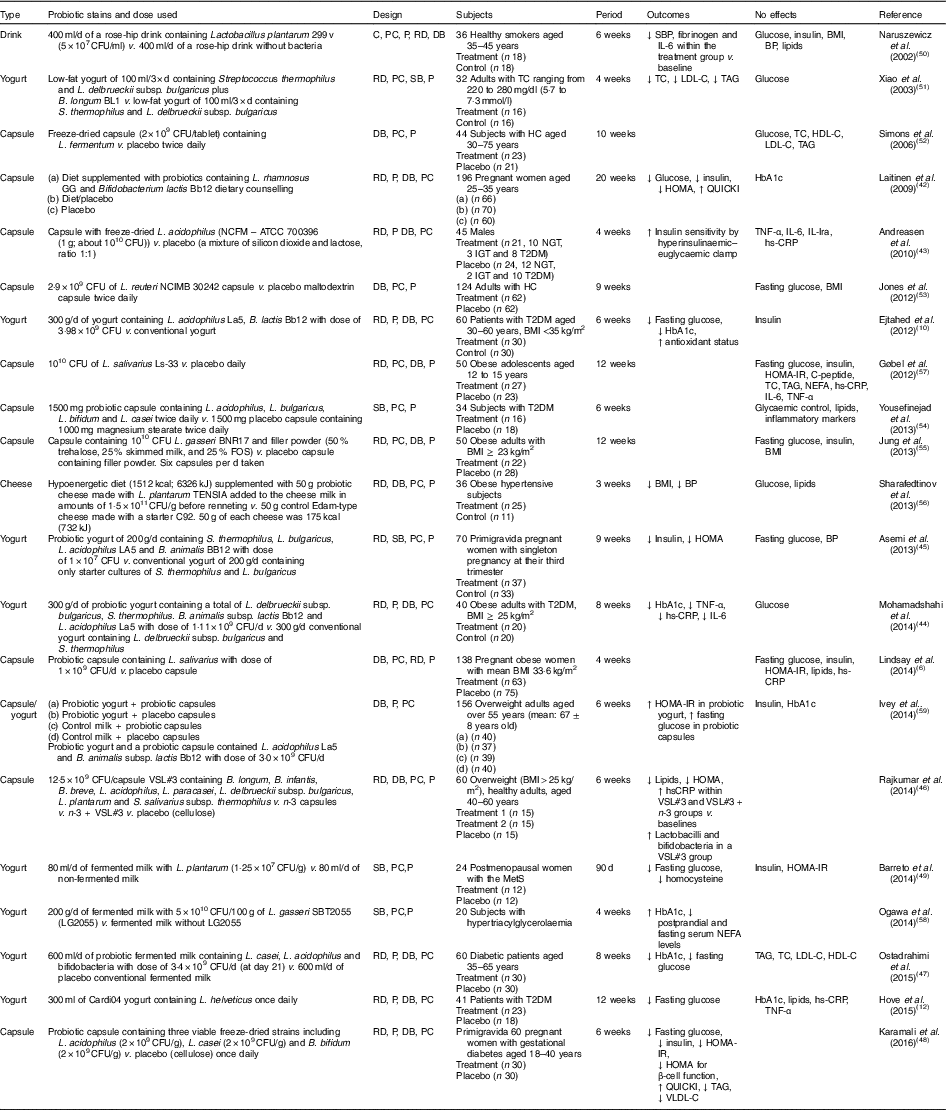
CFU, colony-forming unit; C, control; PC, placebo–control; P, parallel; RD, randomised; DB, double blind; SBP, systolic blood pressure; BP, blood pressure; SB, single blind; TC, total cholesterol; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol; HC, hypercholesterolaemia; HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index; ATCC, American Type Culture Collection; NGT, normal glucose tolerance; IGT, impaired glucose tolerance; T2DM, type 2 diabetes mellitus; IL-Ira, IL-1 receptor antagonist; hs-CRP, high-sensitivity C-reactive protein; HbA1c, glycated Hb; FOS, fructo-oligosaccharide; MetS, metabolic syndrome; VLDL-C, VLDL-cholesterol.
Patients with T2DM supplemented with probiotic yogurt experienced attenuated fasting glucose and glycated Hb (HbA1c) concentrations and increased erythrocyte superoxide dismutase (SOD), glutathione peroxidase (GPx) activities and total antioxidants, compared with the control group( Reference Ejtahed, Mohtadi-Nia and Homayouni-Rad 10 ). Pregnant women given a probiotic supplement (Bifidobacterium lactis Bb12 and Lactobacillus rhamnosus GG) and dietary counselling together had improved glycaemic control during and after pregnancy compared with the control/placebo group( Reference Laitinen, Poussa and Isolauri 42 ).
In sixty women with gestational diabetes, the daily supplement of a probiotic capsule, containing three viable freeze-dried strains including Bifidobacterium bifidum (2×109 CFU/g), Lactobacillus acidophilus (2×109 CFU/g) and L. casei (2×109 CFU/g), for 6 weeks showed improved insulin sensitivity as assessed by the homoeostasis model assessment for insulin resistance (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI), compared with a placebo capsule (cellulose). This probiotic supplementation also lowered TAG and VLDL levels, but this study did not measure HbA1c( Reference Karamali, Dadkhah and Sadrkhanlou 48 ). Moreover, oral supplementation of L. acidophilus NCFM (progenitor of the strain being used for complete chromosome sequencing in order to identify the relationship between genetics and probiotic functionality( Reference Sanders and Klaenhammer 60 )) for 4 weeks improved insulin sensitivity as assessed by hyperinsulinaemic–euglycaemic clamp, compared with a placebo group, without changes in inflammatory markers such as TNF-α, IL-6, IL-Ira and high-sensitivity C-reactive protein( Reference Andreasen, Larsen and Pedersen-Skovsgaard 43 ).
On the other hand, one study of overweight adults( Reference Ivey, Hodgson and Kerr 59 ) has shown conflicting results in that the intake of probiotic yogurt increased HOMA-IR (P=0·038) and probiotic capsules significantly increased fasting glucose (P=0·037) with no change in HOMA-IR( Reference Ivey, Hodgson and Kerr 59 ) (n 77 for the probiotic group and n 79 for the probiotic capsule; the specific study design is shown in Table 1). The probiotics used were Lactobacillus acidophilus La5 and Bifidobacterium animalis subsp. lactis Bb12 (dose of 3·0×109 CFU/d)( Reference Ivey, Hodgson and Kerr 59 ).
A single-blinded clinical trial of thirty-four subjects with T2DM showed no effects on glycaemic control, lipid profiles and inflammatory markers between a placebo group (n 18; 1000 mg magnesium stearate) and a treatment group (n 16) who received 1500 mg probiotic capsules containing Lactobacillus acidophilus, L. bulgaricus, L. bifidum and L. casei twice daily for 6 weeks( Reference Yousefinejad, Mazloom and Dabbaghmanesh 54 ). However, this study provided no counts of probiotics used.
In summary, given the mixed results from human interventions, it is still unclear if probiotics favourably influence glucose control. More human interventions are needed with more comprehensive and dynamic measures of insulin sensitivity, as most studies did not use these. It is also required to investigate the treatment effects of specific strain(s) at different dosages and durations on insulin resistance.
Other fermented food
Kimchi, made with napa cabbage and various ingredients (garlic, red pepper powder, onion, ginger, radish, fermented fish sauce and starch syrup), is a fermented traditional Korean food. Kimchi can give health benefits due to its high nutritional value and abundant bioactive compounds including dietary fibres, minerals, amino acids, vitamins, carotenoids, glucosinolates and polyphenols. Kimchi can be improved by additional ingredients and altered fermentation conditions( Reference Patra, Das and Paramithiotis 61 ). Fermented kimchi mostly contains lactic acid-producing bacteria including Lactobacillus plantarum, Lactobacillus brevis, Pediococcus cerevisiae, Streptococcus faecalis and Leuconostoc mesenteroides, which could exert a probiotic effect( Reference Patra, Das and Paramithiotis 61 ).
Studies have shown beneficial effects of fermented kimchi on glucose metabolism in obese( Reference Kim, An and Lee 62 ) and prediabetic individuals( Reference An, Lee and Jeon 63 ).
Fermented kimchi intake for 4 weeks decreased fasting glucose, fasting insulin, total cholesterol, MCP-1, leptin and the waist:hip ratio compared with fresh kimchi in a cross-over design of twenty-two overweight and obese patients. Fresh kimchi was defined as 1 d-old kimchi and fermented kimchi was defined as 10 d-old kimchi. The number of Lactobacilli in fermented kimchi was higher than in fresh kimchi (4·3×109 (sd 1·2×109)/ml v. 1·4×107 (sd 3×106)/ml)( Reference Kim, An and Lee 62 ). The consumption of fermented kimchi for 8 weeks decreased HbA1c, fasting insulin, HOMA and increased quantitative insulin sensitivity check index (QUICKI) and β-cell function compared with before fermented kimchi intake, in twenty-two adults with prediabetes( Reference An, Lee and Jeon 63 ).
A randomised controlled clinical trial in twenty-four obese women showed that fermented kimchi intake (180 g/d) for 8 weeks altered gut microbiota composition, with a decrease in genus Blautia and an increase in Prevotella and Bacteroides, compared with fresh kimchi intake and up-regulated expression of genes related to the metabolic syndrome such as acyl-CoA synthetase long-chain family member 1 (ACSL1; involved in enhancing fatty acid degradation) and aminopeptidase N (ANPEP; involved in the regulation of pain, angiogenesis, inflammation and apoptosis)( Reference Han, Bose and Wang 64 ).
Very recently, Shin et al. ( Reference Shin, Kang and Jang 65 ) demonstrated metabolic pathways of kimchi action based on in silico modelling of published data. A total of 4351 genes were associated with kimchi metabolites. Of these, 283 genes were associated with carbohydrate metabolism. In all, 309 genes were associated with lipid metabolism and twenty-seven genes (especially GNAS, CTNNB1, EDN1, RAC1 and adenyl cyclases (ADCY1, ADCY2, ADCY5) known to act as regulators of metabolic and cardiovascular function) are directly related with CVD. Twenty-three genes (especially PTPRC, LCK, JAK3, ZAP70 and VEGFA) were related to immune diseases and twenty-five genes were related to endocrine and metabolic diseases( Reference Shin, Kang and Jang 65 ). In summary, these inconsistent findings with probiotic interventions might result from heterogeneity in probiotic strains and populations. Intervention studies should be designed with a specific group and a specific strain( Reference Panwar, Rashmi and Batish 66 ).
Potential mechanisms of action of probiotics
One potential mechanism of anti-diabetic effects is that certain probiotics facilitate production of SCFA (acetate (C2), propionate (C3) and butyrate (C4)), leading to the secretion of incretin hormones which may influence glucose levels( Reference Yadav, Lee and Lloyd 9 , Reference Belenguer, Duncan and Calder 67 ). Yadav et al. ( Reference Yadav, Lee and Lloyd 9 ) have demonstrated a potential mechanism of probiotics through butyrate-induced secretion of glucagon-like peptide 1 (GLP-1) in mouse models. In this study( Reference Yadav, Lee and Lloyd 9 ), VSL#3 consisting of Lactobacillus casei, L. plantarum, L. acidophilus and L. delbrueckii subsp. bulgaricus, Bifidobacterium longum, B. breve and B. infantis and Streptococcus salivarius subsp. thermophilus was used. The levels of the SCFA butyrate in the mouse faecal samples significantly increased after VSL#3 (daily oral dose of 5 mg/kg body weight), as measured by liquid chromatography–electrospray ionisation–tandem MS. Significantly increased plasma butyrate levels were observed in VSL#3-treated mice compared with PBS-treated control mice. For the measurement of butyrate-producing bacteria, gene expression of butyrate kinase was assayed after 2 and 4 weeks of oral administration. Gene expression of butyrate kinase increased at 2 weeks in VSL#3-treated mice. An increase in GLP-1 was observed in the human intestinal L-cell line NCI-H716 treated with butyrate( Reference Yadav, Lee and Lloyd 9 ). GLP-1, an incretin hormone secreted by L-cells mainly in the ileum and large intestine, increases insulin secretion while glucagon is suppressed. GLP1 secretion results in delayed gastric emptying and reduced appetite, food intake and body-weight gain( Reference Drucker and Nauck 68 ). Lactic acid produced by lactic acid-producing bacteria can be converted to acetate or propionate by Clostridium propionicum, Propionibacterium ssp., Desulfovibrio ssp, Veillonella ssp. and Selenomonas ssp.( Reference Seeliger, Janssen and Schink 69 ) via methylmalonyl-CoA or acrylyl-CoA, and then to butyrate via acetyl-CoA by Eubacterium hallii (butyrate-producing species)( Reference Belenguer, Duncan and Calder 67 ).
Other potential mechanisms of anti-diabetic effects of probiotics could be associated with enhanced immunity and increased anti-inflammatory cytokine production, reduced intestinal permeability and reduced oxidative stress( Reference Ejtahed, Mohtadi-Nia and Homayouni-Rad 10 , Reference Yadav, Jain and Sinha 11 , Reference Ma, Forsythe and Bienenstock 70 , Reference Paszti-Gere, Szeker and Csibrik-Nemeth 71 ). In a randomised, double-blind, controlled intervention, patients with T2DM consumed 300 g/d of yogurt (L. acidophilus La5, B. lactis Bb12 with a dose of 3·98×109 CFU) for 6 weeks and experienced a reduction in fasting glucose and HbA1c and an increase in GPx and erythrocyte SOD activities and total antioxidant status, compared with the control (300 g/d of conventional yogurt). GPx and SOD are scavengers of reactive oxygen species( Reference Ejtahed, Mohtadi-Nia and Homayouni-Rad 10 ).
Pre-incubation of HeLa cells with live Lactobacillus reuteri cells for 1–2 h inhibited translocation of NF-κB to the nucleus, inhibited degradation of IKKB (inhibitor of NF-κB kinase subunit β) and prevented expression of pro-inflammatory cytokines under NF-κB regulation. Live L. reuteri up-regulated nerve growth factor and inhibited constitutive synthesis and secretion of IL-8 induced by TNF-α in T84 and HT29 cells (human colonic adenocarcinoma)( Reference Ma, Forsythe and Bienenstock 70 ). Nerve growth factor is known to play roles in the regulation of inflammation( Reference Lambiase, Bracci-Laudiero and Bonini 72 , Reference Ma, Wolvers and Stanisz 73 ) and proliferation of pancreatic β-cells( Reference Pierucci, Cicconi and Bonini 74 ). Metabolites of Lactobacillus plantarum 2142 down-regulated peroxide-induced elevation in proinflammatory cytokines IL-8 and TNF-α in the IPEC-J2 cell line (jejunal epithelia isolated from neonatal piglet)( Reference Paszti-Gere, Szeker and Csibrik-Nemeth 71 ). In streptozotocin-induced diabetic rats, probiotic dahi containing Lactobacillus casei and Lactobacillus acidophilus suppressed streptozotocin-induced oxidative stress in pancreatic tissues by preventing the depletion of glutathione, GPx and SOD, as well as by decreasing thiobarbituric acid-reactive substances and nitrite( Reference Yadav, Jain and Sinha 11 ). This finding implicates that probiotic dahi could delay streptozotocin-induced alteration in glucose homeostasis by exerting an antioxidant effect on β-cells( Reference Yadav, Jain and Sinha 11 ).
Prebiotics and effects of prebiotics on glucose metabolism in human interventions
Prebiotics are non-digestible food ingredients that are not metabolised or absorbed while passing through the upper gastrointestinal tract and are fermented by bacteria in the colon and selectively enhance the growth and/or activity of one or more potential beneficial bacteria (for example, Bifidobacterium and Lactobacillus) in the digestive system( Reference Yoo and Kim 75 – Reference Flesch, Poziomyck and Damin 77 ).
Food sources of prebiotics are seeds, whole grains, legumes, chicory roots, Jerusalem artichokes, onions, garlic and some vegetables. Some prebiotics can be produced during the process of enzymic action or alcohol or cooking( Reference Flesch, Poziomyck and Damin 77 ).
Prebiotics include fructo-oligosaccharides (FOS), galacto-oligosaccharides, lactulose and large polysaccharides (inulin, resistant starches, cellulose, hemicellulose, pectin and gum)( Reference Yoo and Kim 75 ). Of these, researchers have given more attention to FOS( Reference Flesch, Poziomyck and Damin 77 ). Synthetic oligosaccharides such as galacto-oligosaccharies have shown better effects and fewer side effects than natural forms( Reference Flesch, Poziomyck and Damin 77 ). Oligofructose-enriched inulin can act across the whole colon. Oligofructose is a short-chain fructan (a polymer of fructose molecules) containing three to ten monosaccharides linked together. It is quickly fermented and completely metabolised in the ascending part of the colon, whereas inulin is a long-chain fructan containing nine to sixty-four monosaccharides linked together. It is fermented and metabolised in the descending colon( Reference Flesch, Poziomyck and Damin 77 , Reference Dehghan, Pourghassem Gargari and Asghari Jafar-abadi 78 ).
Inulin-type fructans of 10–20 g/d can normalise glucose tolerance or lipid profiles( Reference Sanz and Santacruz 79 – Reference Letexier, Diraison and Beylot 84 ). FOS or inulin of 4 g/d is the minimal requirement for the enhancement of bifidobacteria growth but 14 g/d or more of inulin can cause intestinal discomfort( Reference Flesch, Poziomyck and Damin 77 ).
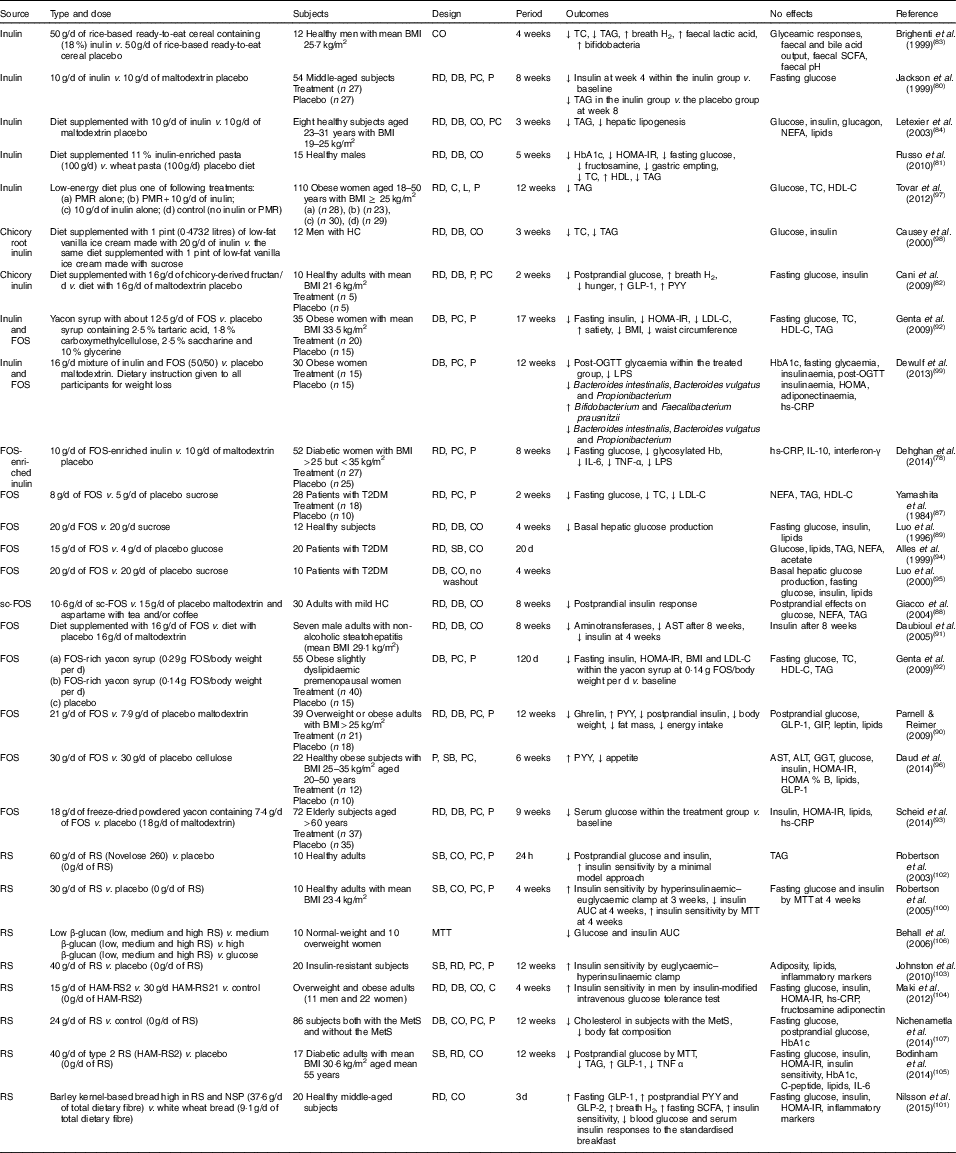
In various animal studies, prebiotics have shown improved glucose metabolism( Reference Kok, Morgan and Williams 85 , Reference Everard, Lazarevic and Derrien 86 ). However, a few human studies have demonstrated inconsistent findings. Human interventions of prebiotics are shown in Table 2.
Table 2 Summary of prebiotic human intervention studies
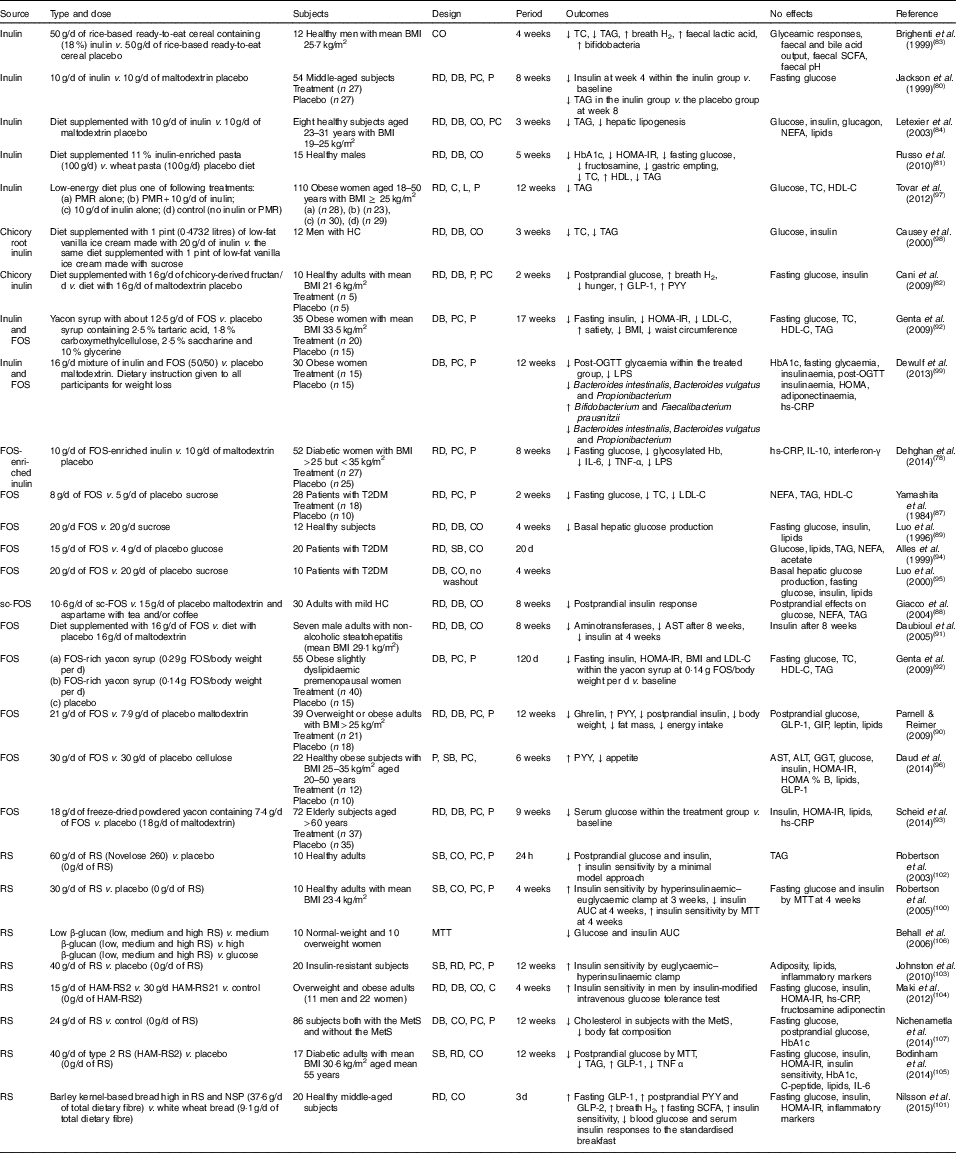
CO, cross-over; TC, total cholesterol; RD, randomised; DB, double blind; PC, placebo–control; P, parallel; HbA1c, glycated Hb; HOMA-IR, homeostasis model assessment of insulin resistance; PMR, partial meal replacement; C, control; L, longitudinal; HDL-C, HDL-cholesterol; HC, hypercholesterolaemia; GLP-1, glucagon-like peptide-1; PYY, peptide YY; FOS, fructo-oligosaccharide; LDL-C, LDL-cholesterol; OGTT, oral glucose tolerance test; LPS, lipopolysaccharide; hs-CRP, high-sensitivity C-reactive protein; T2DM, type 2 diabetes mellitus; SB, single blind; sc-FOS, short-chain fructo-oligosaccharide; AST, aspartate aminotransferase; GIP, glucose-dependent insulinotropic peptide; ALT, alanine aminotransferase; GGT, γ-glutamyltranspeptidase; RS, resistant starch; MTT, meal tolerance test; HAM-RS2, high-amylose maize type 2 resistant starch; MetS, metabolic syndrome.
Fructo-oligosaccharides
Seven studies have shown a favourable effect of FOS( Reference Yamashita, Kawai and Itakura 87 – Reference Scheid, Genaro and Moreno 93 ) on glycaemic control, while three studies of FOS( Reference Alles, de Roos and Bakx 94 – Reference Daud, Ismail and Thomas 96 ) have shown no effect.
Forty-eight overweight or obese adults (BMI>25 kg/m2) in a randomised, double-blind, placebo-controlled trial received 21 g oligofructose per d or a placebo (maltodextrin) for 12 weeks. FOS supplementation decreased ghrelin, glucose and insulin, and increased peptide YY (PYY) compared with a placebo( Reference Parnell and Reimer 90 ). Yamashita et al. ( Reference Yamashita, Kawai and Itakura 87 ) have also demonstrated a beneficial effect of supplementation of 8 g FOS per d for 14 d on glucose metabolism in individuals with T2DM. They found reductions in fasting glucose, total cholesterol and LDL-cholesterol. The intake of short-chain FOS of 10·6 g/d for 2 months reduced postprandial insulin response with no significant alteration in postprandial responses of glucose, NEFA and TAG in mild hypercholesterolaemic adults, compared with placebo( Reference Giacco, Clemente and Luongo 88 ). In a double-blind cross-over design, a daily consumption of 20 g FOS for 4 weeks decreased basal hepatic glucose production with no change in insulin-suppressed hepatic glucose production or insulin-stimulated glucose uptake using a hyperinsulinaemic clamp, compared with a daily consumption of 20 g sucrose in twelve healthy subjects( Reference Luo, Rizkalla and Alamowitch 89 ). However, in subjects with T2DM, supplementation of 20 g FOS had no effect on basal hepatic glucose production, fasting glucose and insulin concentrations( Reference Luo, Van Yperselle and Rizkalla 95 ), and on blood glucose and serum lipids( Reference Alles, de Roos and Bakx 94 ).
Inulin
Effects of inulin on glycaemic control have shown mixed results, with three interventions( Reference Jackson, Taylor and Clohessy 80 – Reference Cani, Lecourt and Dewulf 82 ) showing a positive effect and four interventions( Reference Brighenti, Casiraghi and Canzi 83 , Reference Letexier, Diraison and Beylot 84 , Reference Tovar, Caamano Mdel and Garcia-Padilla 97 , Reference Causey, Feirtag and Gallaher 98 ) showing no effect.
In a parallel study of fifty-four subjects receiving 10 g inulin (n 27) or maltodextrin (n 27) daily for 8 weeks, insulin concentrations were lower at 4 weeks within the inulin group compared with baseline, but no differences were observed at weeks 4 and 8 in comparison with a placebo. No effect of inulin on fasting glucose concentrations was observed compared with a placebo( Reference Jackson, Taylor and Clohessy 80 ). However, in a cross-over study of twelve men with hypercholesterolaemia, a diet supplemented with 1 pint (0·4732 litres) of vanilla ice cream made with 20 g inulin for 3 weeks decreased total cholesterol and TAG but did not alter glucose and insulin, compared with the same diet supplemented with 1 pint of vanilla ice cream made with sucrose( Reference Causey, Feirtag and Gallaher 98 ).
Oligofructose-enriched inulin
Three interventions have shown favourable effects on glycaemic control when a combination of FOS and inulin was used( Reference Dehghan, Pourghassem Gargari and Asghari Jafar-abadi 78 , Reference Genta, Cabrera and Habib 92 , Reference Dewulf, Cani and Claus 99 ). In a randomised controlled study of fifty-two women with T2DM, 10 g FOS-enriched inulin per d (n 27) for 8 weeks lowered fasting glucose and glycosylated Hb and improved inflammatory markers (IL-6, TNF-α) and decreased LPS, compared with a placebo (maltodextrin; n 25)( Reference Dehghan, Pourghassem Gargari and Asghari Jafar-abadi 78 ). Further research is necessary to clarify the effects of oligofructose-enriched inulin on glucose metabolism.
Resistant starch
Consumption of resistant starch improved insulin sensitivity in healthy subjects( Reference Robertson, Bickerton and Dennis 100 – Reference Robertson, Currie and Morgan 102 ) or in subjects with the metabolic syndrome( Reference Johnston, Thomas and Bell 103 , Reference Maki, Pelkman and Finocchiaro 104 ), and lowered postprandial glucose or insulin in individuals with T2DM( Reference Bodinham, Smith and Thomas 105 ) and women( Reference Behall, Scholfield and Hallfrisch 106 ). One study( Reference Nichenametla, Weidauer and Wey 107 ) showed no difference in glycaemic control. The 3 d intake of barley kernel-based bread rich in resistant starch and NSP increased fasting SCFA levels, gut hormones (fasting GLP-1, postprandial PYY and GLP-2) secretion and breath H2 excretion, and improved insulin sensitivity (the Matsuda index) after consuming a standardised breakfast, compared with white wheat bread( Reference Nilsson, Johansson-Boll and Bjorck 101 ).
In summary, the effects of prebiotics (inulin or FOS or oligofructose-enriched inulin administration) on glucose and lipid metabolism are not clear, but resistant starch appears to have a favourable effect on insulin sensitivity.
Other potential prebiotics
Costabile et al. ( Reference Costabile, Klinder and Fava 108 ) suggested that whole-grain wheat could exert a prebiotic effect on gut microbiota composition. This double-blind, randomised, cross-over trial comparing 100 % whole-grain breakfast cereal of 48 g/d with wheat bran breakfast cereal of 48 g/d for 3 weeks showed that whole grain significantly increased the number of faecal bifidobacteria and lactobacilli compared with the wheat bran cereal. However, there were no significant differences in faecal SCFA, fasting glucose, insulin, total cholesterol, TAG or HDL-cholesterol for whole grain intake compared with whole bran( Reference Costabile, Klinder and Fava 108 ).
Potential mechanisms of action of prebiotic-derived SCFA in insulin sensitivity
Microbial fermentation of prebiotics facilitates the production of SCFA (essential endproducts of carbohydrate metabolism) and enhances gut barrier function( Reference Cani, Possemiers and Van de Wiele 109 ). In a mouse model, a prebiotic treatment decreased intestinal permeability and increased GLP-2 secretion, and reducing the hepatic expression of inflammatory and oxidative stress markers and decreasing LPS during obesity and diabetes( Reference Cani, Possemiers and Van de Wiele 109 ).
SCFA and free fatty acid receptors
SCFA in the intestine activate G-protein-coupled receptors (GPR), such as GPR41 (namely, free fatty acid receptor 3; FFAR3) and GPR43 (namely, free fatty acid receptor 2; FFAR2). These receptors are present on ileal and colonic enteroendocrine L-cells, adipocytes and immune cells( Reference Woting and Blaut 110 ). Both GPR41 and GPR43 on intestinal epithelia L-cells trigger the secretion of gut hormones (GLP-1 and PYY). Leptin is also released from adipocytes when SCFA bind to GPR41. PYY, GLP1 and leptin can decrease appetite( Reference Dailey and Moran 111 – Reference Meier and Gressner 114 ). GLP-1 increases insulin secretion from pancreatic β-cells and decreases glucagon secretion from the pancreatic islets, which leads to lower glucose output from the liver and enhanced peripheral uptake of glucose. GLP1 may suppress appetite and food intake via the autonomic nervous system or the brain( Reference Yoo and Kim 75 , Reference Diamant, Blaak and De Vos 115 , Reference Kaji, Karaki and Kuwahara 116 ). It is known that FFAR3 is activated by butyrate and propionate while FFAR2 is activated by acetate and propionate( Reference Layden, Angueira and Brodsky 117 ). FFAR3 knockout mice showed that butyrate and propionate inhibited food intake, reduced high-fat diet-induced weight gain and glucose intolerance and enhanced gut hormone release. FFAR3 was necessary for the maximal induction of GLP-1 by butyrate, whereas FFAR3 was unnecessary for the effects on body weight and glucose-dependent insulinotropic peptide secretion( Reference Lin, Frassetto and Kowalik 118 ). FFAR3 and FFAR2 can be expressed in several cells such as adipocytes, endocrine cells (for example, pancreatic islets) and immune cells( Reference Delzenne and Cani 119 ). FFAR2 is highly expressed in immune cells (neutrophils and monocytes) and haematopoietic tissues, compared with FFAR3( Reference Cox, Jackson and Stanton 120 ). SCFA can exert potent roles in inhibiting lipolysis and inflammation, and regulating energy metabolism( Reference Delzenne and Cani 119 , Reference Cox, Jackson and Stanton 120 – Reference Zaibi, Stocker and O’Dowd 122 ). Ge et al. ( Reference Ge, Li and Weiszmann 121 ) demonstrated that when FFAR2 on adipocytes was activated by SCFA (acetate and propionate), adipocyte lipolysis and differentiation were inhibited, while in GPR43 knockout animals, this was not observed, suggesting that prebiotic fermentation could be detrimental with regard to obesity( Reference Ge, Li and Weiszmann 121 ). However, obese mice and human studies of prebiotics (especially, inulin-type fructans) have not shown this( Reference Dewulf, Cani and Neyrinck 123 , Reference Abrams, Griffin and Hawthorne 124 ). SCFA inhibited the production of MCP-1 and LPS-induced IL-10 in human monocytes, as well as LPS-induced TNF-α and interferon-γ in human peripheral blood mononuclear cells (PBMC: monocytes and lymphocytes (T-cells, B-cells and natural killer cells))( Reference Cox, Jackson and Stanton 120 ).
Anti-inflammatory effects
Elevated pro inflammatory makers such as high-sensitivity C-reactive protein, TNF-α and IL-6 are increased in T2DM( Reference Dehghan, Pourghassem Gargari and Asghari Jafar-abadi 78 ). SCFA can suppress these inflammatory mediators( Reference Cox, Jackson and Stanton 120 , Reference Vinolo, Rodrigues and Hatanaka 125 – Reference Park, Lee and Lee 128 ). SCFA (acetate, propionate, and butyrate) decrease NO( Reference Vinolo, Rodrigues and Hatanaka 125 ). NO is produced by NO synthase which converts oxygen and arginine to citrulline and NO. NO acts as a vasodilator with beneficial effects on vascular health( Reference De Caterina, Libby and Peng 129 – Reference Rudic, Shesely and Maeda 132 ) and has an anti-inflammatory effect under normal physiological conditions( Reference Sharma, Al-Omran and Parvathy 133 ). However, NO participates in immune responses by cytokine-activated macrophages which produce NO in high concentrations( Reference Sharma, Al-Omran and Parvathy 133 ).
SCFA suppressed LPS-stimulated TNF-α( Reference Tedelind, Westberg and Kjerrulf 126 ) from neutrophils and also suppressed TNF-α, IL-1β, IL-6 and NO in RAW 264·7 murine macrophage cells( Reference Liu, Li and Liu 127 ). Moreover, SCFA (0·2–20 mmol/l) lowered the LPS-induced production of TNF-α and interferon-γ in human PBMC in a dose-dependent manner( Reference Cox, Jackson and Stanton 120 ). Butyrate suppressed IL-6 and TNF-α in interferon-γ-stimulated RAW 264·7 murine macrophage cells( Reference Park, Lee and Lee 128 ). Studies( Reference Vinolo, Rodrigues and Hatanaka 125 , Reference Tedelind, Westberg and Kjerrulf 126 , Reference Park, Lee and Lee 128 ) showed that anti-inflammatory effects of SCFA could be mediated by inhibiting the activation of NF-κB (a transcriptional factor involved in the inflammatory response and cell proliferation and TNF-α production( Reference Usami, Kishimoto and Ohata 134 )). Butyrate is a histone deacetylase inhibitor( Reference Usami, Kishimoto and Ohata 134 , Reference Park, Joo and Pedchenko 135 ). SCFA (propionate and butyrate) suppressed the release of LPS-stimulated TNF-α and down-regulated NF-κB by facilitating PGE2 levels and cyclo-oxygenase-2 activities through inhibiting histone deacetylase in PBMC( Reference Usami, Kishimoto and Ohata 134 ) and murine macrophage cell line RAW 264·7 cells( Reference Park, Joo and Pedchenko 135 ). Therefore, SCFA, especially butyrate, exert an anti-inflammatory effect via two potential signalling pathways of NF-κB and histone deacetylase inhibition.
Butyrate also decreased the levels of MCP-1 in a dose-dependent manner with or without LPS in human PBMC( Reference Cox, Jackson and Stanton 120 ). SCFA inhibited the expression of vascular cell adhesion molecule-1 induced by TNF-α and IL-1β in human umbilical vein endothelial cells( Reference Zapolska-Downar, Siennicka and Kaczmarczyk 136 – Reference Zapolska-Downar and Naruszewicz 138 ). Butyrate suppressed T-cell activation stimulated by antigen-presenting cells by down-regulating the expression of intracellular cell adhesion molecule-1 and lymphocyte function-associated antigen-3 in monocytes( Reference Bohmig, Krieger and Saemann 139 ).
SCFA and angiopoietin-like protein 4
Angiopoietin-like protein 4 (ANGPTL4) is a 50 kDa pro-hormone secreted from brown and white adipose tissues, liver, skeletal muscle, intestine and heart. Human ANGPTL4 is mainly expressed in the liver. It is known as fasting-induced adipose factor because ANGPTL4 is up-regulated in white adipose tissue and liver during fasting( Reference Woting and Blaut 110 , Reference Alex, Lichtenstein and Dijk 140 ), while human plasma ANGPTL4 concentrations are reduced after meal consumption( Reference Alex, Lichtenstein and Dijk 140 ). ANGPTL4 is a lipoprotein lipase inhibitor and thus causes decreased uptake of fatty acids into tissue( Reference Woting and Blaut 110 ). In mice, overexpression of ANGPTL4 decreased clearance of TAG-rich lipoproteins and increased circulating TAG levels( Reference Köster, Chao and Mosior 141 ). A very recent study showed that inhibition of or a lower level of ANGPTL4 is associated with lower risk of CVD in mice and non-human primate models( Reference Dewey, Gusarova and O’Dushlaine 142 ). The suppressed ANGPTL4 may result in increased lipoprotein lipase activity and lipolysis( Reference Yoo and Kim 75 , Reference Diamant, Blaak and De Vos 115 ).
On the other hand, lower serum ANGPTL4 levels are observed in subjects with T2DM compared with normal subjects. An inverse association between plasma glucose levels and HOMA-IR, and serum ANGPTL4 levels was found. These findings suggest that decreased ANGPTL4 could play a role in glucose tolerance( Reference Xu, Lam and Chan 143 ).
ANGPTL4 induced by fatty acids via PPAR in various tissues appears to reduce cellular lipid overload, oxidative stress and inflammation( Reference Lichtenstein, Mattijssen and de Wit 144 , Reference Georgiadi, Lichtenstein and Degenhardt 145 ). The SCFA, especially propionate, inhibited lipid synthesis in the presence of acetate as a source of acetyl-CoA in hepatocytes( Reference Demigné, Morand and Levrat 146 ). Propionate and/or butyrate, but not acetate, activated ANGPTL4 production in intestinal (Caco-2, HT-29 and HCT-116) and hepatic (HepG2) cancer cell lines( Reference Grootaert, Van de Wiele and Van Roosbroeck 147 ) and the entero-endocrine cell line HuTu-80 from the human small intestine( Reference Alex, Lichtenstein and Dijk 140 ).
ANGPTL4 is a downstream target gene of PPAR( Reference Yoo and Kim 75 ). PPAR, transcription factors with three isoforms (α, β and γ) are a superfamily of nuclear receptors( Reference Ferré 148 ). Fatty acids and lipid-derived substrates are their ligands. PPAR-γ agonists are used as T2DM treatment drugs. PPAR-α, present in liver, heart and skeletal muscle, promotes primarily hepatic fatty acid oxidation, ketone body synthesis and glucose sparing, while PPAR-γ, expressed in the lower intestine, adipose tissue and immunity cells, facilitates an increase in fatty acid storage in adipocytes( Reference Ferré 148 ).
Thiazolidinediones (TZD) are strong activators of PPAR-γ which improve insulin sensitivity and facilitate insulin-mediated suppression of gluconeogenesis in the liver and glucose uptake in the skeletal muscle( Reference Ferré 148 , Reference Bouskila, Pajvani and Scherer 149 ). However, PPAR-γ is expressed in adipose tissues but not in muscle, the main insulin-sensitive tissue( Reference Ferré 148 ). Activation of PPAR-γ causes release of adiponectin from mature adipocytes, which stimulates AMP involved in the up-regulation of glucose transporters (especially, GLUT4) in skeletal muscle, the stimulation of increased fatty acid oxidation in mitochondria( Reference Ferré 148 ), as well as the down-regulation of gluconeogenesis in the liver( Reference Rutter, Da Silva Xavier and Leclerc 150 ), consequently leading to improved insulin sensitivity in skeletal muscle( Reference Ferré 148 ) and in the liver( Reference Bouskila, Pajvani and Scherer 149 ). Metformin, a T2DM treatment medicine, is a stimulator of AMPK( Reference Ferré 148 ). It is suggested that the combined use of PPAR ligands (for example, TZD) and SCFA could minimise weight gain from TZD releasing ANGPTL4( Reference Korecka, De Wouters and Cultrone 151 ).
SCFA and intestinal gluconeogenesis
One potential mechanism for SCFA to prevent T2DM involves intestinal gluconeogenesis (IGN) which is mediated by signalling of the periportal nervous system( Reference De Vadder, Kovatcheva-Datchary and Goncalves 152 ). Hepatic gluconeogenesis and IGN play opposite roles in glucose homeostasis. IGN might be inversely associated with the risk of T2DM, as beneficial effects of IGN on a reduction in food intake, weight gain and hepatic glucose output, and on improvement in glycaemic control, have been shown( Reference Troy, Soty and Ribeiro 153 – Reference Gautier-Stein, Zitoun and Lalli 155 ). In contrast, increased hepatic gluconeogenesis is related to the risk of T2DM( Reference DeFronzo 156 , Reference Magnusson, Rothman and Katz 157 ). The intestine produces approximately 20–25 % of total endogenous glucose in the fasted state( Reference Mithieux 158 ). Glucose produced by the intestine is sent to the portal vein. The periportal neural system in the portal vein walls detects glucose and sends a signal to the brain for the modulation of energy and glucose metabolism( Reference Mithieux 158 ). Interestingly, butyrate directly promotes IGN gene expression in enterocytes by increasing intracellular cyclic AMP levels in an FFAR2-independent manner( Reference De Vadder, Kovatcheva-Datchary and Goncalves 152 ). Propionate binding to FFAR3 present in the portal nerves increases IGN gene expression through a portal hypothalamic neural circuit( Reference De Vadder, Kovatcheva-Datchary and Goncalves 152 ). The benefit of this gut–brain neural circuits has been shown for portal glucose sensing initiated by a protein-enriched diet, resulting in decreased food intake( Reference Mithieux, Misery and Magnan 154 ).
IGN-deficient mice (with disruption of the glucose 6-phosphatase (G6Pase) catalytic subunit in the intestine) fed a SCFA- or FOS-rich diet showed no favourable effect on glucose and insulin with no change in body weight, compared with normal mice fed a SCFA- or FOS-rich diet( Reference De Vadder, Kovatcheva-Datchary and Goncalves 152 ). Moreover, normal mice fed a high-fat/high-sucrose diet supplemented with FOS showed improved glucose and insulin tolerance and decreased fat mass, whereas these metabolic benefits were absent in IGN-deficient mice fed the same diet with FOS( Reference De Vadder, Kovatcheva-Datchary and Goncalves 152 ). Therefore, IGN appears to be essential for the effect of SCFA on glucose homeostasis.
Synbiotics and effects of synbiotics on glucose metabolism in human interventions
A combination of probiotics and prebiotics is described as a synbiotic( Reference Flesch, Poziomyck and Damin 77 ). Lactobacillus acidophilus DSM20079 induced 14·5-fold more butyrate in the presence of inulin or pectin than in the presence of glucose( Reference Nazzaro, Fratianni and Nicolaus 159 ).
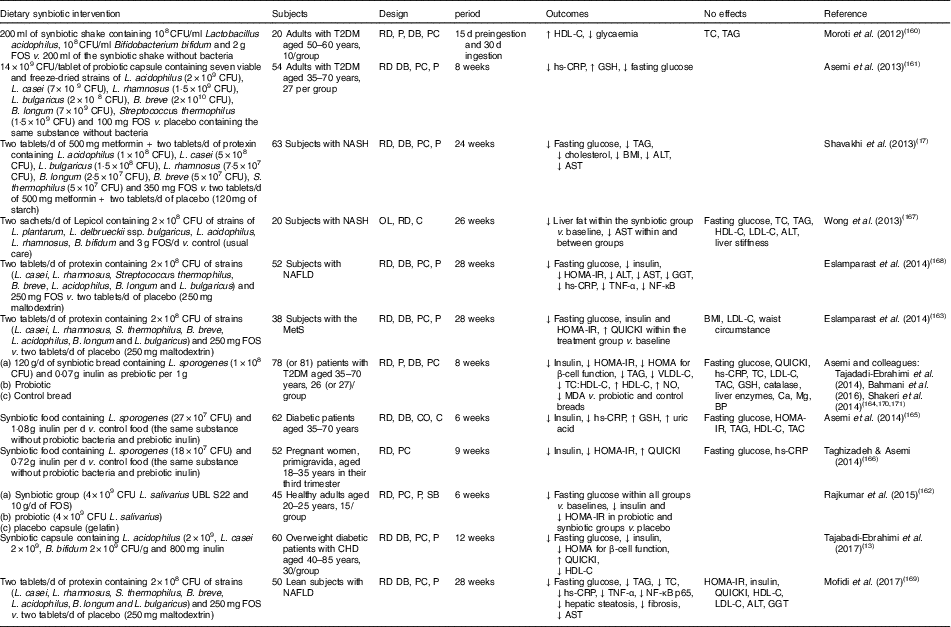
Human interventions of synbiotics are shown in Table 3. Eleven of twelve studies of synbiotics have shown favourable effects on glucose metabolism( Reference Tajabadi-Ebrahimi, Sharifi and Farrokhian 13 , Reference Shavakhi, Minakari and Firouzian 17 , Reference Moroti, Souza Magri and de Rezende Costa 160 – Reference Mofidi, Poustchi and Yari 169 ). Three( Reference Shavakhi, Minakari and Firouzian 17 , Reference Eslamparast, Poustchi and Zamani 168 , Reference Mofidi, Poustchi and Yari 169 ) of four( Reference Shavakhi, Minakari and Firouzian 17 , Reference Wong, Won and Chim 167 – Reference Mofidi, Poustchi and Yari 169 ) studies in subjects with non-alcoholic fatty liver disease have shown a positive effect on glucose control. The Asemi research group conducted a randomised, double-blind, placebo-controlled trial in subjects with T2DM; the consumption of synbiotic bread (containing the probiotic Lactobacillus sporogenes (1×108 CFU) and 0·07 g inulin per 1 g as prebiotic) for 8 weeks improved insulin metabolism, lipid profiles and plasma NO and malondialdehyde levels, compared with the probiotic alone (Lactobacillus sporogenes; 1×108 CFU) and a control bread( Reference Tajadadi-Ebrahimi, Bahmani and Shakeri 164 , Reference Bahmani, Tajadadi-Ebrahimi and Kolahdooz 170 , Reference Shakeri, Hadaegh and Abedi 171 ).
Table 3 Summary of synbiotic human intervention studies
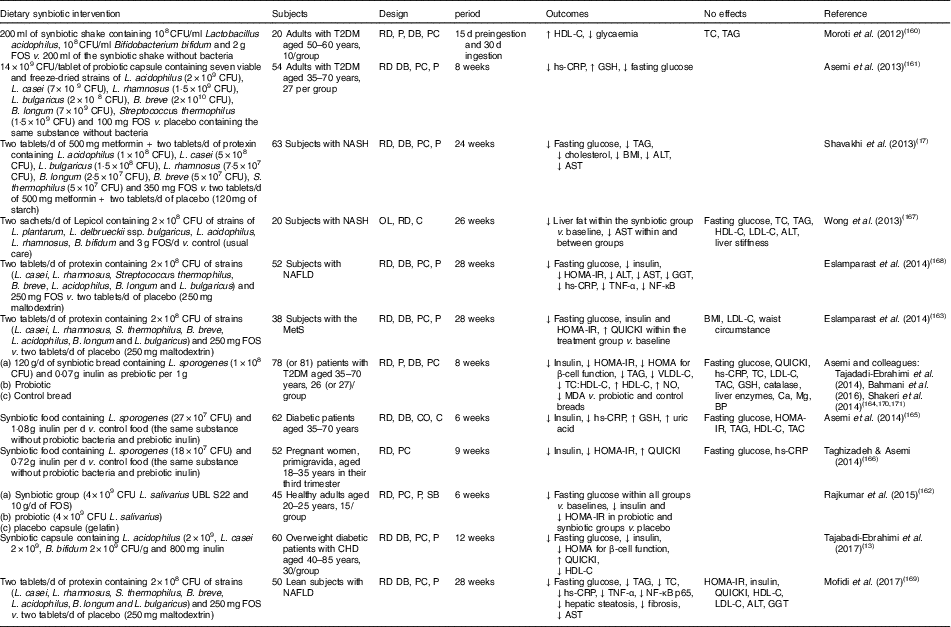
CFU, colony-forming unit; FOS, fructo-oligosaccharide; T2DM, type 2 diabetes mellitus; RD, randomised; P, parallel; DB, double blind; PC, placebo–control; HDL-C, HDL-cholesterol; TC, total cholesterol; hs-CRP, high-sensitivity C-reactive protein; GSH, glutathione; NASH, non-alcoholic steatohepatitis; ALT, alanine aminotransferase; AST, aspartate aminotransferase; OL, open label; C, control; LDL-C, LDL-cholesterol; NAFLD, non-alcoholic fatty liver disease; HOMA-IR, homeostasis model assessment of insulin resistance; GGT, γ-glutamyltransferase; MetS, metabolic syndrome; QUICKI, quantitative insulin sensitivity check index; VLDL-C, VLDL-cholesterol; MDA, malondialdehyde; TAC, total antioxidant capacity; BP, blood pressure; CO, cross-over; SB, single blind.
In summary, a very limited number of interventions have shown beneficial effects on glucose metabolism. More pronounced effects of synbiotics on glycaemic control and inflammation have been observed than with the use of probiotics alone( Reference Jumpertz, Le and Turnbaugh 15 ).
Conclusion
Individuals with obesity or T2DM have been observed to have a different composition of gut microbiota. Altered gut microbiota may contribute to the development of T2DM. The composition of gut microbiota can be beneficially modified by probiotics and/or prebiotics to maintain glucose homeostasis. The potential mechanisms of action could involve insulinotropic and satiety effects mediated by gut hormones, GLP-1 and PYY, a β-cell-protective effect by reduced oxidative stress and lowered pro-inflammatory cytokines, anti-lipolytic activities and enhanced insulin sensitivity via GLUT4 through the up-regulation of AMPK signalling in tissues. An additional role of SCFA is in glycaemic control through IGN and mediated by the periportal nervous system. The antidiabetic effects of SCFA require further research. Use of resistant starch and synbiotics may become a diabetic nutritional strategy. Overall human interventions of probiotic and prebiotics showed mixed findings, so further work is required.
Acknowledgements
P. M. C. is supported by a National Health and Medical Research Council (NHMRC) Principal Research Fellowship, University of South Australia. Y. A. K. is supported by an Australian Postgraduate Award.
All authors conceived of the manuscript structure and contributed to the writing and editing.
The authors have no conflicts of interest related to this paper.