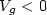Review Article
Research Article
Near-field optical imaging of noble metal nanoparticles
-
- Published online by Cambridge University Press:
- 13 August 2004, pp. 3-18
-
- Article
- Export citation
High dielectric constant oxides
-
- Published online by Cambridge University Press:
- 02 December 2004, pp. 265-291
-
- Article
- Export citation
Editorial
Research Article
Kinetic modeling of low-pressure nitrogen discharges and post-discharges
-
- Published online by Cambridge University Press:
- 03 November 2004, pp. 125-152
-
- Article
- Export citation
Nanomaterials and Nanotechnologies
Research Article
Fine modulations in the diffraction pattern of boron nitride nanotubes synthesised by non-ablative laser heating
-
- Published online by Cambridge University Press:
- 30 August 2004, pp. 293-300
-
- Article
- Export citation
Organic Materials and Devices
Research Article
Modelling of twisted optical waveguides with edge elements
-
- Published online by Cambridge University Press:
- 03 November 2004, pp. 153-157
-
- Article
- Export citation
Semiconductors and Devices
Research Article
Effect of the hydrogen dilution on the short-range and intermediate-range-order in radiofrequency magnetron sputtered hydrogenated amorphous silicon films
-
- Published online by Cambridge University Press:
- 25 June 2004, pp. 19-26
-
- Article
- Export citation
Organic Materials and Devices
Research Article
Fowler-Nordheim current modeling of metal/ultra-thin oxide/semiconductor structures in the inversion mode, defects characterization*
-
- Published online by Cambridge University Press:
- 25 June 2004, pp. 27-41
-
- Article
- Export citation
Nanomaterials and Nanotechnologies
Research Article
Electrostatic displacement of multiwalled carbon nanotubes by scanning a voltage-applied tip of an atomic force microscope
-
- Published online by Cambridge University Press:
- 23 November 2004, pp. 301-304
-
- Article
- Export citation
Surfaces, Interfaces and Films
Research Article
Titanium carbide film deposition on silicon wafers by pulsed KrF laser ablation of titanium in low-pressure CH4 and C2H2 atmospheres
-
- Published online by Cambridge University Press:
- 25 August 2004, pp. 159-163
-
- Article
- Export citation
Interaction of very low energy electron beams with micro porous silicon substrates: ESD studies of H− ion desorption
-
- Published online by Cambridge University Press:
- 03 November 2004, pp. 165-171
-
- Article
- Export citation
Molecular dynamics simulations of palladium cluster growth on flat and rough graphite surfaces
-
- Published online by Cambridge University Press:
- 25 June 2004, pp. 43-50
-
- Article
- Export citation
Magnetism, Superconductivity and Related Devices
Research Article
Magnetostatic interactions in bilayer films
-
- Published online by Cambridge University Press:
- 25 August 2004, pp. 305-311
-
- Article
- Export citation
Surfaces, Interfaces and Films
Research Article
Microstructure and morphology evolution in chemical solution deposited semiconductor films: 2. PbSe on As face of GaAs(111)
-
- Published online by Cambridge University Press:
- 25 June 2004, pp. 51-57
-
- Article
- Export citation
Plasma, Discharges and Processes
Research Article
Superhard nanocomposites: design concept, properties, present and future industrial applications*
-
- Published online by Cambridge University Press:
- 05 August 2004, pp. 313-317
-
- Article
- Export citation
Nanomaterials and Nanotechnologies
Research Article
Non-uniformity of temperatures along nanotubes in hot reactors and axial growth
-
- Published online by Cambridge University Press:
- 30 August 2004, pp. 173-178
-
- Article
- Export citation
Plasma, Discharges and Processes
Research Article
Removal of pollutants by plasma catalytic processes*
-
- Published online by Cambridge University Press:
- 23 November 2004, pp. 319-324
-
- Article
- Export citation
Surfaces, Interfaces and Films
Research Article
Characteristics of deposited Eu2O3 film as a thick gate dielectric for silicon
-
- Published online by Cambridge University Press:
- 25 August 2004, pp. 59-64
-
- Article
- Export citation
Laser, Optics, Optoelectronics and Nanophotonics
Research Article
More about the light beam shaping by the integration method
-
- Published online by Cambridge University Press:
- 05 August 2004, pp. 179-186
-
- Article
- Export citation
Plasma, Discharges and Processes
Research Article
Effects of CO2 molecules upon and argon thermal plasma
-
- Published online by Cambridge University Press:
- 05 August 2004, pp. 187-195
-
- Article
- Export citation
Self-absorbing method to measure the population of the metastable levels in an argon microwave plasma at atmospheric pressure*
-
- Published online by Cambridge University Press:
- 23 November 2004, pp. 325-330
-
- Article
- Export citation