Stomach cancer – also known as gastric cancer – is a major public health problem in many countries. In 2019, there were over 1·2 million newly diagnosed cases of stomach cancer worldwide. It is the fifth most commonly diagnosed cancer globally and the third leading cause of cancer mortality(Reference Bray, Ferlay and Soerjomataram1). In 2019, stomach cancer was responsible for almost a million deaths and accounted for over 22 million disability-adjusted life years (DALY)(2). The largest share of this cancer burden occurred in low-income and middle-income countries, where most healthcare systems are ill equipped to provide the complex care that is required.
Vietnam is among the top ten countries with the highest rates of stomach cancer worldwide(Reference Bray, Ferlay and Soerjomataram1,2) . Stomach cancer is the third most frequently diagnosed cancer in Vietnam, and in 2018, over 17 500 new cases of stomach cancer were diagnosed in the country. It is also the third leading cause of cancer mortality in Vietnam(Reference Sung, Ferlay and Siegel3). The 5-year survival rate of people with stomach cancer is very poor, typically ranging between 20 and 30 %(2,4) . With such grim prognosis, primary prevention is crucial, especially in low-income and middle-income countries.
High-salt (Na) consumption is thought to increase the risk for stomach cancer via multiple mechanisms. Studies in animals have demonstrated that chronic exposure to high-salt intake alters the viscosity of the mucous lining of the stomach, progressively causing atrophic gastritis and intestinal metaplasia. In addition, high-salt concentrations facilitate Helicobacter pylori colonisation of the stomach, which then upregulates the expression of the carcinogenic protein (cagA)(Reference Gaddy, Radin and Loh5). Exposure to high-salt diets also promotes the formation of N-nitroso compounds, which are well-known human carcinogens associated with gastrointestinal cancers(Reference Loh, Jakszyn and Luben6,Reference Keszei, Goldbohm and Schouten7) . Pooled evidence from prospective cohort studies has demonstrated that high-salt consumption significantly increases the risk of developing stomach cancer(Reference D’Elia, Rossi and Ippolito8,Reference Fang, Wei and He9) . A recent dose–response meta-analysis by Fang and colleagues found that a 5 g/d increment in dietary salt intake increased the risk of stomach cancer by 12 %(Reference Fang, Wei and He9). Given very high average dietary salt intake globally, this translated to attribution of well over a third (38·2 %) of the stomach cancer DALY to diets high in Na in the recent Global Burden of Disease (GBD) analysis(2).
The most recent WHO STEPS survey in Vietnam estimated that adults consume on average 9·4 g of salt/d(10), which is about double the current WHO recommendation of <5 g/d(11). Existing research on the harmful effects of high-salt intake globally(Reference He, Tan and Ma12) and in Vietnam(Reference Aminde, Phung and Phung13,Reference Taylor, Hoek and Deltetto14) has mostly focused on its impact on blood pressure and CVD. The impact of such high-salt diets on the burden of stomach cancer has not been evaluated in Vietnam. Recognising high-salt consumption as a major public health concern, the government of Vietnam in their recent 2018 Vietnam Health Program has set national average salt intake targets to be achieved by 2025 and by 2030(15). Building on the above evidence gaps, the aim of this study was to (1) quantify the avoidable burden of stomach cancer (absolute and relative changes in incidence and mortality) and (2) estimate the impact of these changes in stomach cancer burden on healthy life years (LY), if the Vietnam Health Program and WHO salt reduction targets were attained.
Methods
Salt reduction policy target
The 2018 Vietnam Health Program has as main aim to improve the well-being, longevity and quality of life of Vietnamese people in the 2018–2030 period. One of the three goals of this program is to ‘Ensure proper nutrition and enhance physical activity to improve the stature and health for the people’. In line with this goal, national salt reduction policy targets were set which included: a reduction in current salt intake to less than 8 g/d by 2025 and to further reduce salt intake to less than 7 g/d by 2030(15). In this study, we attempted to quantify the potential impact of these salt policy targets on the future burden of stomach cancer and population health. We implemented this by comparing the attainment of these national salt policy targets and the WHO (5 g/d) salt reduction target to a business-as-usual setting, where current consumption patterns persist into the future.
Study design, population and data sources
We conducted a simulation study using a multi-cohort proportional multistate lifetable Markov model. This model combines demographic, epidemiological and economic data to project the impacts of interventions over the lifecourse and is well-suited for population-level chronic disease modelling given its capacity to account for comorbidities(Reference Briggs, Wolstenholme and Blakely16,Reference Barendregt, Van Oortmarssen and Van Hout17) . A closed cohort of all adult Vietnamese aged ≥ 25 years (total of 61 million people, 48·4 % men) alive in 2019 were included in this analysis.
Age- and gender-specific data on salt intake were obtained from the 2015 Vietnam WHO STEPwise approach to non-communicable disease (NCD) surveillance (STEPS) study(10). This nationally representative survey included adults aged 18 to 69 years from 4651 households across all sixty-three provinces in Vietnam. Spot urine samples were collected from participants to measure urinary Na (salt) content. The INTERSALT formulae(Reference Brown, Dyer and Chan18) were then used to estimate 24-h urinary Na excretion from the spot urine samples, and these were used as a proxy for Na intake in this study. Due to the absence of reliable data, we assumed that people ≥ 70 years had similar salt intake levels to those aged 69 years. For baseline stomach cancer epidemiology, age- and sex-specific data were obtained from the 2019 GBD study. Model input parameters are provided in the appendix.
Modelling framework
We developed a proportional multistate lifetable Markov model, a dynamic epidemiological model that simulated age- and sex-stratified cohorts of the Vietnamese population ≥ 25 years alive in 2019 over their remaining lifetime. The model contains three distinct but linked sections: a risk factor (exposure) section, a standard cause-elimination lifetable and a section for each disease being modelled with an independent disease progression (Markov) or illness-death process(Reference Barendregt, Van Oortmarssen and Van Hout17). The age- and sex-stratified disease-specific morbidity and mortality rates for the disease(s) being modelled (stomach cancer), together with ‘all other’ morbidity and mortality rates (for diseases not included in the model) feed into the lifetable for the calculation of all-cause or total morbidity and all-cause mortality rates in the main lifetable. The model simulates two main populations – a reference (business-as-usual) population with current risk factor (salt intake) distribution, baseline disease (stomach cancer) epidemiology and population demographics and an identical population that receives the ‘intervention’, that is, a change in the risk factor (reduced salt intake).
Modelling salt intake and stomach cancer risk
Dietary salt intake was modelled as a continuous variable with a lognormal distribution. This distribution was used to model salt intake levels assuming the right skew of salt intake data and the implausibility of less than or zero salt intake levels. The age- and sex-stratified population salt distributions were shifted from current levels towards achieving the mean intake levels stipulated in the national Vietnam Health Program (8 g/d by 2025 and 7 g/d by 2030) and the WHO recommendation (5 g/d) by 2030. Relative risk estimates of the direct link between salt intake and stomach cancer risk were obtained from the GBD 2019 comparative risk assessment study(19). For the main analysis, and consistent with the GBD study framework, we considered the optimal salt distribution or theoretical minimum risk exposure level (TMREL) to be 3 g of Na (∼7·6 g of salt) and varied this to 1 g and 5 g of Na in the sensitivity analysis(20). By integrating the exposure distribution and the risk function in the potential impact fraction – which calculates the proportional change in a disease condition following a change in the exposure or risk factor(Reference Barendregt and Veerman21), we estimate the post-intervention incidence of stomach cancer, see Box 1.
Box 1: Potential impact fraction formula and post-intervention incidence calculation
where x = salt intake levels, RR (x) = relative risk, P (x) = baseline salt intake distribution by age and sex, P* = salt distribution after the intervention, i = start integration level (0·5 g of salt), j = end integration level (25 g of salt), dx = integration with respect to x, with i and j as the integration boundaries.
The potential impact fraction is calculated for each sex and 5-year age group using the respective salt distributions and relative risks.
The formula: I’ = I * (1-PIF) , where I’ = incidence after intervention, I = baseline incidence; is used to calculate the post-intervention stomach cancer incidence.
Stomach cancer state transition and lifetable modelling
The stomach cancer state-transition model contains four states: healthy (alive and free from stomach cancer), diseased (alive with stomach cancer), dead from stomach cancer and dead from other causes. Annual probabilities of proportions of the population transiting between these states is influenced by incidence, remission and case fatality hazards (Fig. 1). The potential impact fraction described above feeds into the ‘intervention’ section of the stomach cancer state-transition model to estimate post-intervention incidence, which influences the prevalence and then mortality rates. The latter feed into the ‘intervention’ lifetable, alter the all-cause mortality rates and propagate through to estimate LY(Reference Barendregt, Van Oortmarssen and Van Hout17). These LY are adjusted for poor quality of life to obtain health-adjusted life years (HALY). This quality-of-life adjustment is obtained by dividing the age- and sex-stratified years lived with disability (YLD) for stomach cancer by the prevalence of stomach cancer, and then adjusted for background disability using all-cause population YLD. The sex-stratified 5-year age cohorts of the population are simulated through annual cycles until everyone reaches 100 years or dies. The impact of the intervention is determined by the difference in incidence, mortality and HALY between the reference (business-as-usual) and the intervention (salt reduction policy targets achieved) populations.
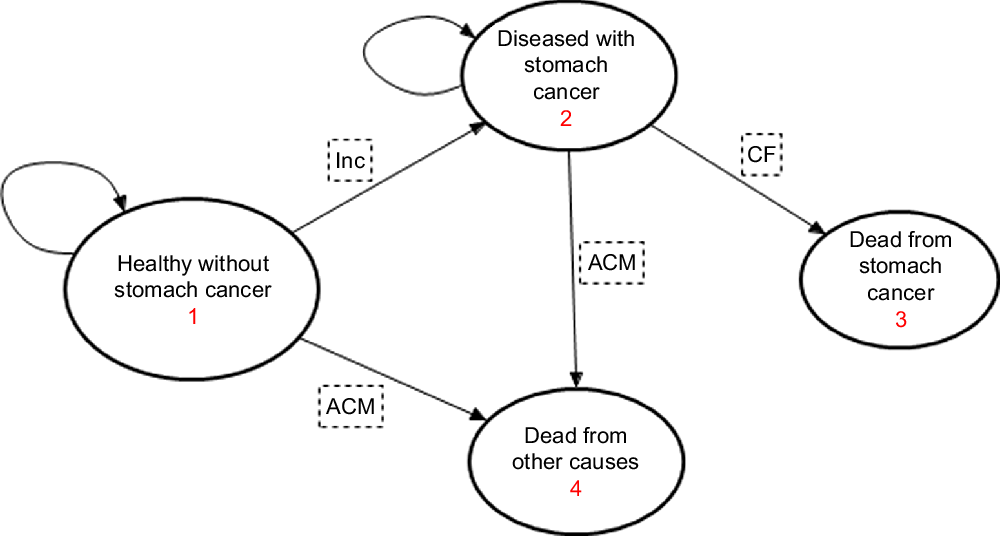
Fig. 1 State-transition diagram showing the four health states for the stomach cancer Markov model. Inc = probability of stomach cancer incidence, CF = case fatality probability, ACM = all-cause mortality probability. Straight arrows imply for each annual cycle, the progression (transition) of proportions of the population from each sex and age-group cohort to the next state, while the circular arrows imply probability of remaining in the same state
Scenarios and sensitivity analysis
In the base-case analysis, we quantified the changes in health outcomes following a gradual (linear) reduction from current salt consumption level (9·4 g/d) to (i) an average of 8 g/d by 2025 (National Target (NT) 1); (ii) an average of 7 g/d by 2030 (NT 2); and (iii) an average of 5 g/d as recommended by WHO by 2030 (WT). In additional scenarios, we modelled lifetime impacts, that is, the national and WHO targets are achieved and sustained for the remaining lifetime of the population. Next, we modelled intervention decay scenarios. These included: NT 1 levels are achieved and sustained for the first 5 years (until 2030) followed by a gradual phasing out of the intervention effect modelled as a linear reduction in impact (by -25 % every 5 years) until zero impact beyond the year 2050 (akin to salt intake in intervention population is back to baseline levels). Similarly, NT 2 and WT are achieved and sustained for the first 5 years (until 2035) followed by a linear reduction in effect (by -25 % every 5 years) until zero impact beyond the year 2055. Figure 2 depicts all the nine population salt intake scenarios that were modelled. Furthermore, current evidence appears unsettled on what the optimal level or TMREL of Na (salt) intake should be, that is, the level of consumption at which disease risk is the lowest(20). Thus, consistent with the GBD study, we used a TMREL of 3 g of Na (∼7·6 g of salt) in our base-case analysis and conducted one-way sensitivity analyses using 1 g and 5 g of Na as optimal levels(19,20) .
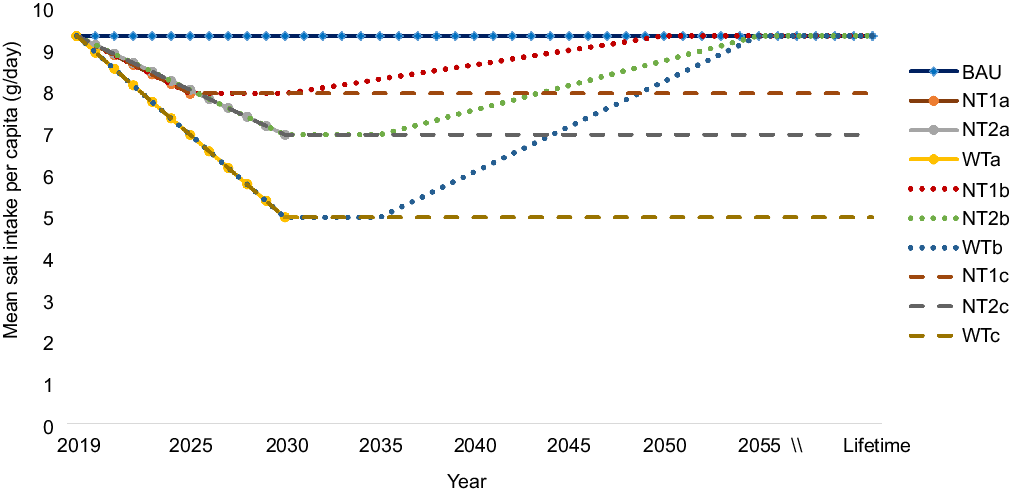
Fig. 2 Modelled scenarios of reduction in overall mean population salt intake. BAU (business as usual), where baseline salt intake levels remain unchanged into the future; National target of 8 g/d salt intake achieved by 2025; NT2a, National target of 7 g/d salt intake achieved by 2030; WTa, WHO target of 5 g/d achieved by 2030; NT1b, National target of 8 g/d achieved by 2025 and sustained to 2030 and effect gradually phased out to zero impact beyond 2050; NT2b, National target of 7 g/d achieved by 2030 and sustained to 2035, then effect gradually phased out to zero impact beyond 2055; WTb, WHO target of 5 g/d achieved by 2030 and sustained to 2035, then effect gradually phased out to zero impact beyond 2055; NT1c, National target of 8 g/d achieved and effect sustained over lifetime; NT2c, National target of 7 g/d achieved and effect sustained over lifetime; WTc, WHO target achieved and effect sustained over lifetime
Uncertainty analyses
To assess parameter uncertainty in our model, we specified appropriate distributions for key input parameters: salt intake levels (lognormal distribution) and relative risks (lognormal distribution). Probabilistic sensitivity analyses using second-order Monte Carlo simulations were conducted to jointly capture the overall uncertainty of key parameters in our outcome projections. We ran 2000 simulations and report the 95 % uncertainty intervals (UIs) (2·5th and 97·5th percentile) around our best estimates. The simulations were implemented using the Ersatz 1·35 software (Epigear International)(Reference Barendregt22). To reflect the Vietnam Health Program policy timelines and also to quantify the long-term impacts, outcomes are reported over three time horizons: 6 years (2019 to 2025), 11 years (2019 to 2030) and lifetime (2019 till the entire cohort dies or reaches 100 years).
Results
Estimated population salt reduction
We modelled a gradual linear reduction in average population salt intake from the 2019 base year level to the policy target levels. For 25–30-year-old men, this was an estimated mean salt reduction of 0·383 g/annum (to reach an average of 8 g/d by 2025), 0·300 g/annum (to reach an average of 7 g/d by 2030) and 0·482 g/annum (to reach an average of 5 g/d by 2030). Corresponding estimates for women were 0·033 g, 0·109 g and 0·291 g/annum, respectively. Table 1 in the supplementary file depicts the baseline salt intake and the estimated average age- and sex-stratified annual reductions modelled.
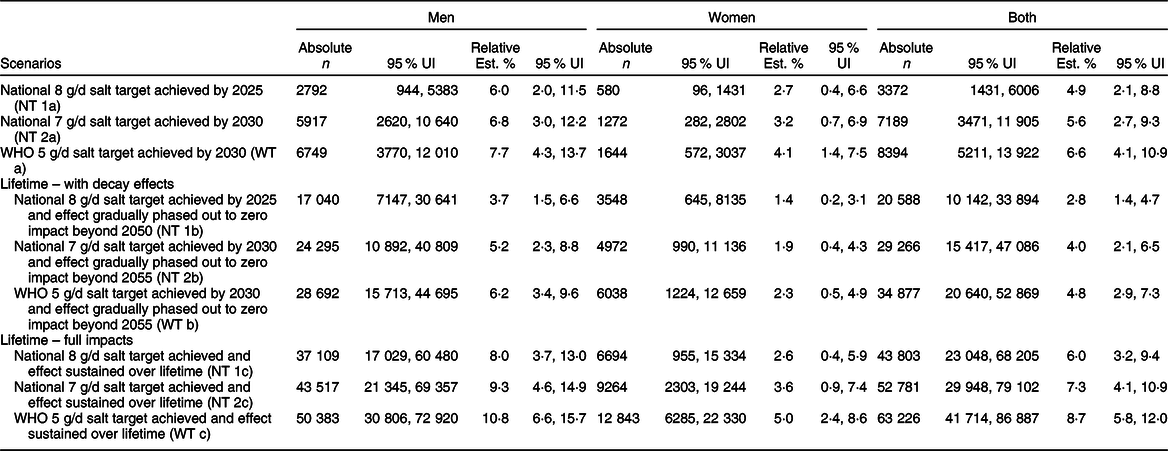
Table 1 Estimated reductions in incidence of stomach cancer in Vietnam between 2019 to 2030 and over the lifetime
UI, Uncertainty interval.
Changes in incidence of stomach cancer by 2025 and 2030
Our model estimates that compared with business as usual where current population salt intake levels persist, a gradual reduction in salt intake over 6 years (between 2019 and 2025) to an average of 8 g/d, could prevent about 3372 (95 % UI: 1431, 6006) new cases of stomach cancer by 2025 (relative reduction in incidence: 6 % in men v. 2·7 % in women). Over 11 years, that is, by 2030, about 7189 (95 % UI (3471, 11 905)) new cases of stomach cancer could be prevented if the 7 g/d national policy were achieved (relative reduction in incidence: 6·8 % in men v. 3·2 % in women). Furthermore, if the WHO 5 g/d target was attained by 2030, there could be 8394 (95 % UI (5211, 13 922)) fewer incident cases of stomach cancer (relative reduction in incidence: 7·7 % in men v. 4·1 % in women) (Table 1).
Changes in mortality from stomach cancer by 2025 and 2030
Over a period of 6 years (2019–2025), our model estimates that there could be 1875 (95 % UI (691, 3482)) fewer deaths from stomach cancer (relative reduction in mortality: 2·9 % in men v. 1·5 % in women), if the mean population salt intake decreased linearly to 8 g/d. Over a period of 11 years, that is, by 2030, achieving the 7 g/d policy target could avert 4851 (95 % UI (2153, 8453)) stomach cancer deaths (relative reduction in mortality: 4·4 % in men v. 2·2 % in women). Moreover, if the WHO 5 g/d salt target was achieved by 2030, about 5855 (95 % UI (3255, 9709)) stomach cancer deaths could be averted (relative reduction in mortality: 5·2 % in men v. 2·8 % in women) (Table 2).
Table 2 Estimated reduction in mortality from stomach cancer in Vietnam between 2019 to 2030 and over the lifetime

UI, uncertainty interval.
Estimated gains in healthy life years by 2025 and 2030
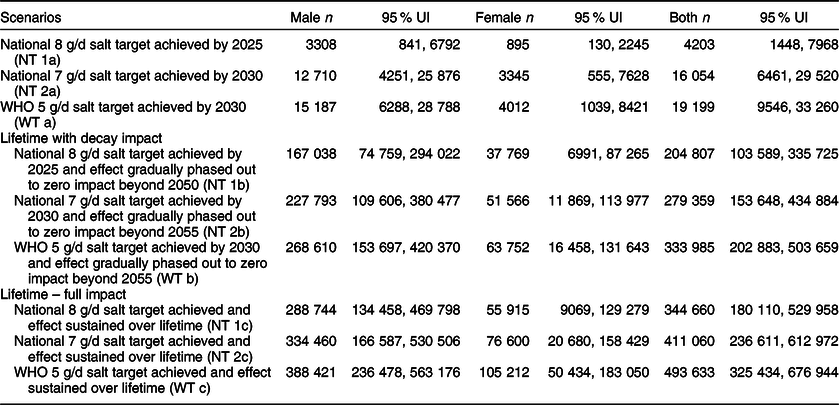
The above epidemiological changes in stomach cancer burden if the average 8 g/d salt target was reached were projected to accrue 3308 (95 % UI (841, 6792)) HALY for men and 895 (95 % UI (130, 2245)) HALY for women by 2025. If the 7 g/d salt target were reached, this would lead to 12 710 (95 % UI (4251, 25 876)) HALY gained for men and 3345 (95 % UI (555, 7628)) HALY gained for women by 2030. Furthermore, if adult Vietnamese reduced their average salt intake to the WHO 5 g/d target, up to 15 187 (95 % UI (6288, 28 788)) HALY and 4012 (95 % UI (1039, 8421)) HALY could be gained for men and women, respectively, by 2030 (Table 3).
Table 3 Estimated health-adjusted life years (HALY) gained between 2019 and 2030 and over the lifetime of adults in Vietnam
UI, uncertainty interval.
Lifetime impacts and sensitivity analyses
Compared with business-as-usual, if the 8 g/d by 2025, the 7 g/d by 2030 and 5 g/d by 2030 salt targets were achieved and sustained over the population lifecourse (i.e. from 2019 until the entire cohort died or reached 100 years of age), our model projects that overall, about 43 803 (95 % UI (23 048, 68 205)), 52 781 (95 % UI (29 948, 79 102)) and 63 226 (95 % UI (41 714, 86 887)) incident stomach cancer events, respectively, could be prevented. Corresponding stomach cancer deaths that could be averted were 39 274 (95 % UI (20 579, 61 220)), 47 289 (95 % UI (26 849, 70 753)) and 56 673 (95 % UI (37 412, 77 950)), respectively.
In decay scenarios with a gradual phase-out in salt reduction effects towards with nil effect beyond 2050 (for the 8 g/d target) and 2055 (for 7 g/d and 5 g/d targets), our model estimated 20 588 (95 % UI (10 142, 33 894)), 29 266 (95 % UI (15 417, 47 086)) and 34 877 (95 % UI (20 640, 52 869)), respectively, for incident stomach cancers prevented. For stomach cancer mortality, the corresponding number of deaths averted were 18 983 (95 % UI (9307, 31 334)), 26 892 (95 % UI (14 137, 43 340)) and 32 108 (95 % UI (18 927, 48 991)).
With respect to healthy LY, our model estimates that an extra 344 660 (for the 8 g/d target), 411 060 (for the 7 g/d target) and over 493 633 (for the 5 g/d target) stomach cancer-related HALY could be gained. In the decay scenarios with a gradual phase out of the policy effects, the corresponding estimates were 204 807 HALY, 279 359 and 333 985 HALY gained (see Tables 1, 2 and 3).
In one-way sensitivity analysis, compared with a TMREL of 3 g of Na (base case), using an optimal intake or TMREL of 1 g of Na resulted in larger health gains in the order of 4–7 % for the 8 g/d target by 2025 and by up to 9–17 % (for the 7 g/d target by 2030) and 23–44 % (for the WHO target by 2030) compared with base case. In the lifetime scenarios, using an optimal intake of 1 g of Na increased health gains observed by 4–22 % relative to base case for the national targets and up to 63 % for the WHO target. On the other hand, applying a higher TMREL of 5 g of Na compared with 3 g (base case), considerably reduced the health benefits by 63–79 % for all scenarios and time horizons (see Fig. 3 and Table 4) .
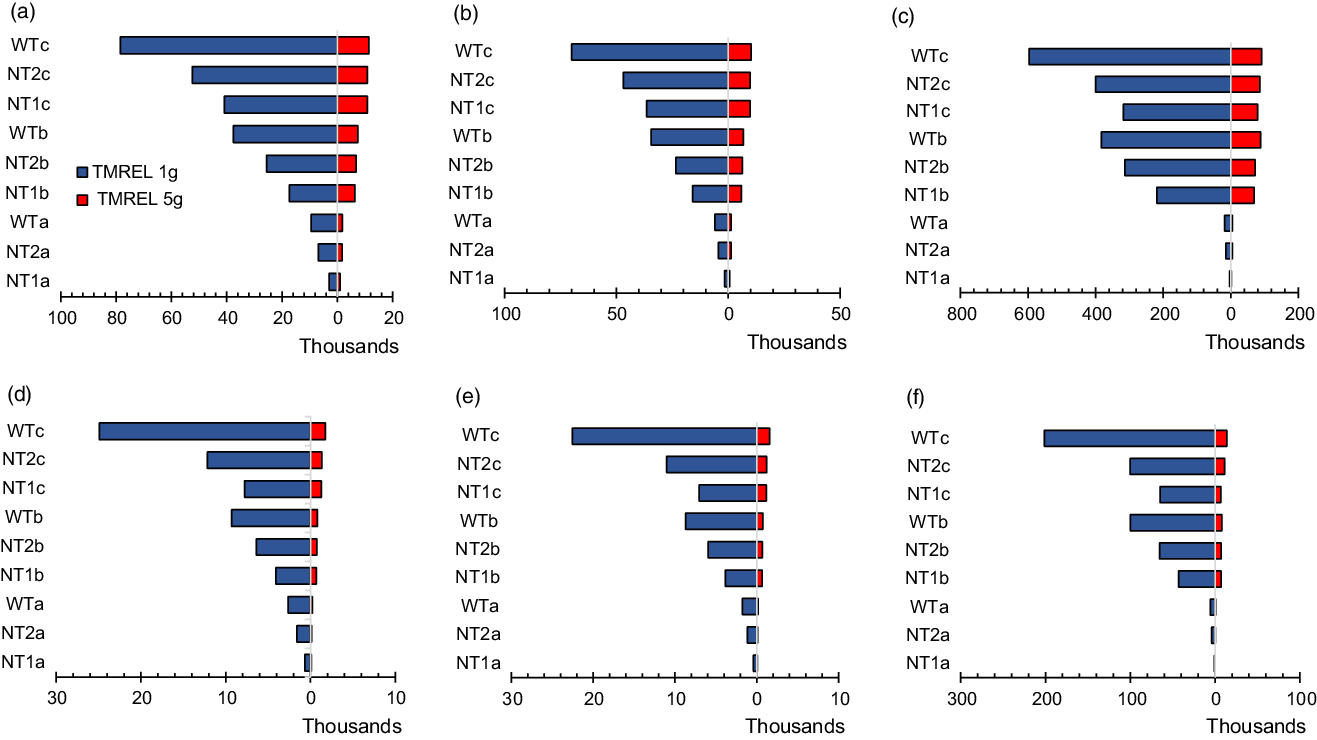
Fig. 3 Tornado plot of one-way sensitivity analysis showing the impacts of different TMREL on incidence, mortality and HALY over varied time horions. Panels A (incidence), B (mortality) and C (HALY) for men and panels D (incidence), E (mortality) and F (HALY) for women. TMREL, Theoretical minimum risk exposure level (optimal level of Na consumption at which disease risk is lowest; base case: 3 g of Na (∼7·6 g of salt), sensitivity analysis: 1 g of Na (∼2·5 g of salt) and 5g Na (∼12·6 g of salt); NT1a, National target of 8 g/d salt intake achieved by 2025; NT2a, National target of 7 g/d salt intake achieved by 2030; WTa, WHO target of 5 g/d achieved by 2030; NT1b, National target of 8 g/d achieved by 2025 and sustained to 2030 and effect gradually phased out to zero impact beyond 2050; NT2b, National target of 7 g/d achieved by 2030 and sustained to 2035, then effect gradually phased out to zero impact beyond 2055; WTb, WHO target of 5 g/d achieved by 2030 and sustained to 2035, then effect gradually phased out to zero impact beyond 2055; NT1c, National target of 8 g/d achieved and effect sustained over lifetime; NT2c, National target of 7 g/d achieved and effect sustained over lifetime; WTc, WHO target achieved and effect sustained over lifetime
Table 4 Comparison of relative changes in base case estimates from varying the TMREL for sodium in the univariate sensitivity analysis
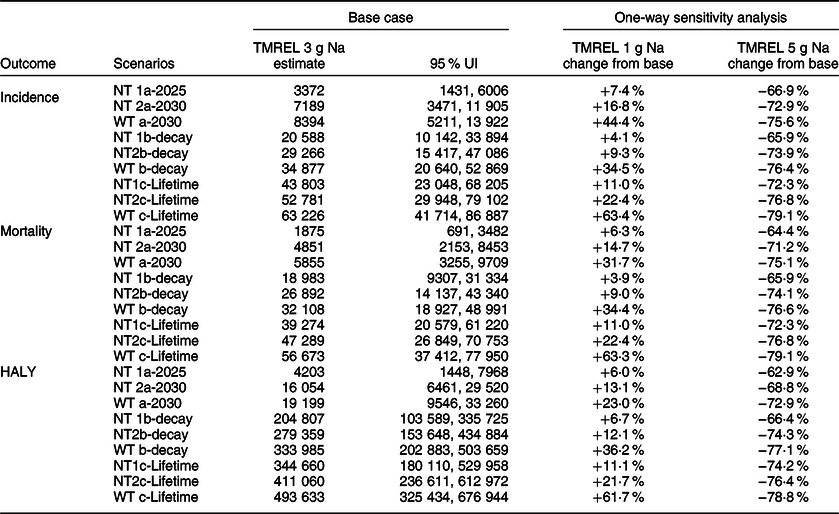
NT1a, National target of 8 g/d salt intake achieved by 2025; NT2a, National target of 7 g/d salt intake achieved by 2030; WTa, WHO target of 5 g/d salt intake achieved by 2030; NT1b, National target of 8 g/d salt intake achieved by 2025 and effect gradually phased out to zero impact beyond 2050; NT2b, National target of 7 g/d achieved by 2030 and effect gradually phased out to zero impact beyond 2055; WTb, WHO target of 5 g/d achieved by 2030 and effect gradually phased out to zero impact beyond 2055; NT1c, National target of 8 g/d achieved and effect sustained over lifetime; NT2c, National target of 7 g/d achieved and effect sustained over lifetime; WTc, WHO target achieved and effect sustained over lifetime; TMREL, theoretical minimum risk exposure level; UI, uncertainty interval.
Discussion
In this study, we estimate the potential impact of achieving the 2018 Vietnam Health Program salt reduction targets on the future burden of stomach cancer and population health. Compared with current levels of salt consumption, about 3400 incident cases of and 1900 deaths from stomach cancer could be prevented if the 8 g/d target was achieved over six years, that is, by 2025. Achieving the average 7 g/d target over 11 years (by 2030) could avert 7200 incident cases and about 4800 deaths from stomach cancer. These changes in stomach cancer burden translated to 4200 and 16 000 HALY gained by 2025 and 2030, respectively. Larger impacts in avoidable disease burden could be obtained with further reductions in salt intake down to the WHO target of 5 g/d, for which we estimate over 5800 stomach cancer deaths prevented or postponed and about 19 200 HALY gained by 2030. Similarly, if these reductions in salt consumption to the national and WHO targets were sustained over the remaining lifetime, even bigger health gains could be attained. Our results were sensitive to varied optimal levels of salt intake. As expected, when compared with 3 g of Na, larger benefits were obtained with an optimal intake level 1 g of Na as opposed to considerable shrinking when a higher optimal intake level of 5 g of Na was implemented.
Very few studies (mostly from high-income countries) have quantified the long-term impacts of excess salt intake on the burden of stomach cancer. Kypridemos et al. modelled various scenarios in relation to the well-known United Kingdom 2003–2011 salt reduction program. This program included public awareness campaigns, food labelling and voluntary reformulation of processed foods by food industries. In a scenario where the decline in salt intake observed in the program continued to 2015 (that is, 8·9 g/d in 2003 to 7·1 g/d in 2015), an estimated 5000 incident cases of stomach cancer and 2000 fewer deaths from the disease were prevented over that period(Reference Kypridemos, Guzman-Castillo and Hyseni23). Further salt reductions to ∼6 g/d by 2030 were estimated to result in an additional 1200 new stomach cancer cases prevented and 700 deaths postponed. Another recent modelling study in the USA has shown that compared with status quo, a low-Na DASH diet could reduce the lifetime risk of stomach cancer by 24·8 % in men and 21·2 % in women. In addition, this prevented twenty-seven and fourteen stomach cancer cases per 10 000 people, respectively, for men and women and postponed twenty-four stomach cancer deaths in men and thirteen stomach cancer deaths in women per 10 000 persons(Reference Kim, Oh and Truong24). Despite differences in modelling approaches, with Kypridemos et al. using dynamic microsimulation and the latter study using Markov models, the results from these studies are akin to our findings – demonstrating the substantial avoidable stomach cancer burden from population-wide salt reduction.
Our results show that benefits were significantly greater in men than in women. This could be explained by several factors. First, men had much higher baseline salt intake and hence needed a greater absolute salt reduction to meet the program targets modelled. Second, men have higher incidence (nearly twice for some age-groups) and slightly higher fatality rates (in older ages) for stomach cancer compared with women. The high hazard rates in men are consistent with the recent 2020 GLOBOCAN report(Reference Sung, Ferlay and Siegel3). It is in part explained by the clustering of other major risk factors for gastric cancer such as smoking and heavy alcohol use in men. Put together, it is highly likely that men would benefit more from primary prevention interventions that modify population exposures by shifting the distributions of major risk factors like salt intake to healthy ranges.
Public health and policy implications
Over two-thirds of adult Vietnamese reported that they consume just the right amount of salt. However, estimates from urinary Na excretion indicate actual intake are nearly twice that recommended by the WHO(10). As most (70 %) of the salt consumed in Vietnam is added during cooking or at the table and from fish sauces(10,25) , strategies to achieve the Healthy Vietnam Program salt reduction targets need to focus on consumers. Do and colleagues evaluated a suite of behavioural change interventions including mass media, school interventions and community programs conducted for a year and found significant reductions in Na intake in a province in Vietnam(Reference Do, Santos and Trieu26). Such interventions could be scaled nation-wide across all sixty-three provinces to achieve larger returns. Recently, the government of Vietnam through the Department of Preventive Medicine of the Ministry of Health in collaboration with the WHO country office and support from Vital Strategies organisation have commenced a nation-wide media campaign to educate the public on the ills of excess salt intake and the need to reduce its consumption(25). The long-term sustainability of these programs is unknown. However, recent modelling assessing the lifetime impacts of media campaigns and school-based programs for salt reduction found that despite the modest effects, these interventions could deliver large population health gains and were cost saving if effects are sustained(Reference Aminde, Cobiac and Veerman27).
For Vietnam and other low-income and middle-income countries where most of the salt is added during cooking, another viable intervention for consideration is replacing regular salt with low-Na potassium-rich salt-substitutes. Evidence from large pragmatic community trials have shown that they are very effective in reducing blood pressure and vascular events(Reference Bernabe-Ortiz, Sal and Ponce-Lucero28,Reference Neal, Wu and Feng29) , as well as being highly cost-effective(Reference Aminde, Cobiac and Veerman27). Nonetheless, given the expansion of multinational food industries in many low-income and middle-income countries, partly fuelling the nutrition transition with growing consumption of ultra-processed foods rich in salt, sugar and fats, these governments will need to consider multi-pronged strategies. Among others, the inclusion of upstream interventions like industry food reformulation, food labelling using consumer-friendly methods that are easy to interpret like traffic light warnings, would be needed(Reference Hyseni, Elliot-Green and Lloyd-Williams30). These measures must be accompanied by regular surveillance, monitoring and evaluation to assess progress and identify areas needing attention to ensure sustained and equitable impacts.
Recent analysis of the burden of gastric cancer showed that the age-standardised incidence and death rates have significantly declined since 1990. However, the rate of decline appears to have slowed down and plateaued in recent years, especially in East Asia and Pacific region(2,Reference Yang, Zhang and Zhang31) . It is suggested that this decline may partially be explained by declining trends in the global prevalence of smoking (another major risk factor for stomach cancer)(Reference Yang, Zhang and Zhang31). We posit that the recent plateau (and reversal for some countries in Asia) in the trend might in part be driven by increasing salt consumption that has accompanied the nutrition transition in Asia(Reference Zhang, Dhakal and Zhao32,Reference Baker, Machado and Santos33) , which has the highest salt consumption levels globally(20). Moreover, the GBD study showed that while about 38·2 % of global age-standardised stomach cancer-related DALY were attributable to diets high in salt (smoking was second contributor at 24·5 %), a much higher fraction (61·3 %) was observed in East Asia, approximately twice that of other regions(2). This demonstrates the substantial contribution that high-salt diets in the region make to the stomach cancer-related morbidity and mortality.
During the past two decades, the government of Vietnam has made commitments towards cancer control, for example, setting up the National Cancer Control Program as part of the national strategy for the prevention and control of NCD 2015–2025(34). However, a recent review highlighted a number of shortcomings including limited availability and accessibility to diagnostic and treatment services across some provinces; inadequate funding (2·5–3·5 % of total health expenditure) for NCD programs despite NCD accounting for 70 % of all deaths; low community awareness with nearly four-fifths of patients diagnosed at advanced stages of cancer and limitations in data infrastructure(Reference Pham, Bui and Kim35). The high lethality of stomach cancer, limited capacity and associated healthcare costs underscore the need for primary prevention to take precedence in efforts to combat this condition. Simple effective and cost-effective strategies like population dietary salt reduction should therefore be high on the policy agenda for measures to mitigate the burden of stomach cancer in Vietnam, Asia and globally.
Strengths and limitations
Our study has some limitations. First, our modelling does not consider the future trends in salt consumption. Given the ongoing nutrition transition, salt intake may increase over time. It is also possible that consumption might decline in response to the recent government initiatives. Predicting the balance of this is not immediately straightforward; however, our analysis reveals that with the current high-salt consumption, even very modest reductions in salt intake are likely to deliver sizeable health gains. Second, we do not explicitly model the lag time from exposure to incidence of stomach cancer. This implies our estimates maybe on the high end; however, in our lifetime scenarios, we model intervention decays down to zero impact beyond 20 years, which mitigates any overestimation. Third, we model a closed cohort of adult Vietnamese. Thus, our lifetime health outcomes do not capture the benefits of salt reduction that accrue from younger cohorts as they age, rendering our results conservative.
Despite the above, our study has some strengths. To our knowledge this study is the first from Asia to estimate the burden of stomach cancer that could be avoided from dietary salt reduction. Our findings contribute to bridging the knowledge gap in the region and provides important evidence needed by policy makers as they prioritise strategies to tackle cancer and NCD. Furthermore, by quantifying the differential impact of varied TMREL, we demonstrate that irrespective of the optimal salt intake level considered, substantial gains in population health could be obtained. Finally, we model multiple time horizons including lifecourse, which goes beyond the current national targets and thereby provides comprehensive evidence on the long-term impacts of policy actions or inactions. Finally, by using local survey data on salt consumption, we allay any issues with transferability of evidence from elsewhere, given that most of the evidence on future impacts of salt intake on stomach cancer is from high-income countries.
Conclusion
Our study suggests that achieving the Healthy Vietnam program salt reduction targets could avert a substantial proportion of the future stomach cancer burden in Vietnam by 2025–2030 and gain thousands of healthy LY. Larger health benefits could be obtained if the WHO salt reduction target was met and targets sustained over the lifespan. Given the high lethality of stomach cancer and limited diagnostic and therapeutic resources in Vietnam, investing in primary prevention strategies such as salt reduction should be high on the policy agenda to optimise population health and alleviate future pressure on the health system.
Acknowledgements
Acknowledgements: None. Financial support: This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Authorship: L.N.A., H.N.P. and J.L.V. were involved in the conceptualisation of the research. L.N.A. conducted the modelling analysis and wrote the first draft manuscript. J.L.V., L.J.C., D.P. and H.N.P. provided critical revisions. All authors interpreted the findings, read and approved the final manuscript. Ethics of human subject participation: Ethics approval was not required for this study as there was no direct contact with patients, and it used freely available secondary data.
Conflicts of interest:
There are no conflicts of interest.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S136898002200177X










