Introduction
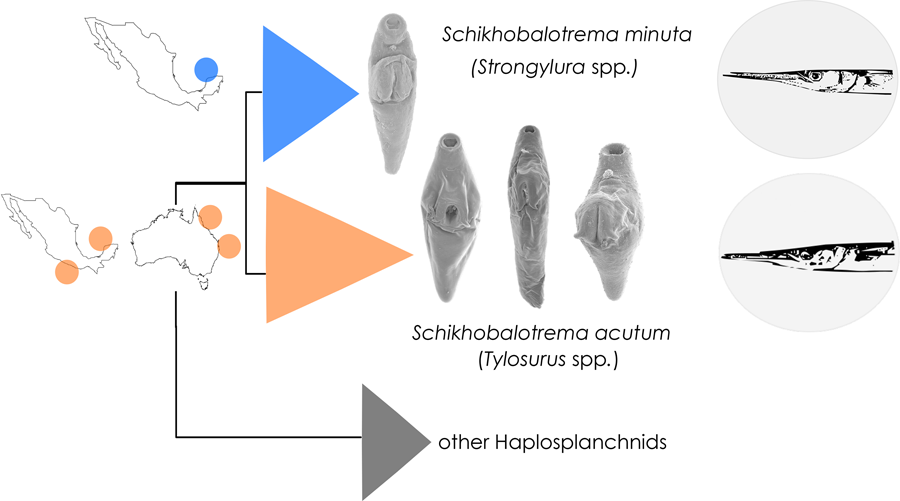
Trematodes of the family Haplosplanchnidae Poche, 1926 infect the digestive tract of a diverse array of marine fishes across the globe (Madhavi, Reference Madhavi, Jones, Bray and Gibson2005), with the group predominantly infecting herbivorous fishes (Huston et al., Reference Huston, Cutmore and Cribb2018). The traditional classification of the family considers species to be allocated into 4 subfamilies; however, based on recent phylogenetic analyses the subfamilies are not currently recognized by some authors (Huston et al., Reference Huston, Cutmore and Cribb2018). The family comprises 10 genera, of which the most speciose is Schikhobalotrema Skrjabin & Guschanskaja, 1955 with 26 valid species (Huston et al., Reference Huston, Cutmore and Cribb2017; WoRMS, 2023). Schikhobalotrema was proposed for Deradena acutum Linton, Reference Linton1910, a parasite of 2 species of needlefish (Belonidae) from the Gulf of Mexico (GoM). While there have been a further 2 species described from belonids, like the rest of the Haplosplanchnidae, the members of Schikhobalotrema have overwhelmingly been described and reported from herbivorous fishes (particularly those of the families Acanthuridae, Hemiramphidae, Mugilidae, Pomacentridae and the scarine Labridae). In the most recent review of the genus, Huston et al. (Reference Huston, Cutmore and Cribb2017) proposed a new species from 2 species of belonids off the eastern coast of Australia, and argued that the lack of molecular information for members of this genus, and the fact that the constituent species possess few complex morphological characters, makes the delimitation of species of Schikhobalotrema a difficult task.
While studying the trematode fauna of marine and estuarine fishes of Mexico, specimens of Schikhobalotrema were sampled from the intestines of belonids, namely Tylosurus pacificus (Steindachner) from off Chamela Bay and from off Barra de Coyuca, Acapulco on the eastern Pacific (EP) coast, Tylosurus acus off Celestún and Strongylura marina and Strongylura notata from La Carbonera coastal lagoon in northern Yucatán, GoM. In this study, we analyse the distribution of Schikhobalotrema species occurring in belonids over a wide geographical range that includes the tropical Indo-west Pacific (IWP), the tropical EP, and the GoM, using morphological and molecular data. Following the paradigm recently proposed for the recognition of marine fish trematode species by Bray et al. (Reference Bray, Cutmore and Cribb2022) we document the convincingly wide distribution for the type-species and describe a new species of Schikhobalotrema from estuarine needlefishes of the genus Strongylura van Hasselt from La Carbonera coastal lagoon in Yucatán, GoM.
Materials and methods
Host collection and morphological study
Specimens of the Pacific agujon, T. pacificus, were obtained from commercial fisheries from 2 localities on the Pacific coast of Mexico: off Chamela Bay, Jalisco state in 1994; and off Barra de Coyuca, Acapulco, Guerrero state in 2018. Specimens of the agujon needlefish, T. acus, were obtained from a commercial fishery off the coast of Celestún, Yucatán in 2019, and specimens of the redfin needlefish, St. notata and the Atlantic needlefish, St. marina were collected from lagoons on the coast of Yucatán in the GoM in 2022. Fishes were dissected, the gastrointestinal tract removed, placed in Petri dishes with 0.85% saline solution and observed under a stereomicroscope. Trematodes morphologically identified as belonging to Schikhobalotrema were recovered alive and fixed in 2 different ways. Specimens from off Chamela Bay were fixed under slight coverslip pressure with Bouin's fluid and placed in vials with 70% ethyl alcohol (EtOH). Specimens from off Barra de Coyuca, Acapulco and from Yucatán were killed with nearly boiling 0.85% saline solution; some specimens were fixed without pressure in 10% formalin for morphological examination, and some were placed in vials with 100% EtOH for molecular analysis.
Specimens were stained with Mayer's paracarmine or Gomori's trichrome, dehydrated in a graded ethanol series, cleared in methyl salicylate and mounted as permanent slides in Canada balsam for morphological study. Specimens were observed using an Olympus BX51 light microscope equipped with differential interference contrast; drawings were made using a drawing tube attached to the same microscope. Measurements are expressed in micrometres, with the range followed by the mean in parentheses. Measurements of specimens from off Chamela Bay were not combined with those from the other specimens because the specimens were flattened. Specimens were deposited at the Colección Nacional de Helmintos (CNHE), Instituto de Biología, Mexico City. Two specimens from off Barra de Coyuca, Acapulco, 1 from off Celestún and 1 from La Carbonera lagoon were prepared for scanning electron microscopy (SEM). Specimens for SEM were dehydrated in a graded ethanol series, critical point dried and mounted on a strip of carbon conductive tape. Samples were sputter coated with gold and observed in a Hitachi Stereoscan Model SU1510 (Hitachi Ltd, Tokyo, Japan) at 10 kV.
Additionally, our analysis included specimens sampled from Australia in the form of paragenophores of samples incorporated in the description of Schikhobalotrema huffmani by Huston et al. (Reference Huston, Cutmore and Cribb2017). Samples were collected from the hound needlefish, Tylosurus crocodilus, from off Lizard Island, Great Barrier Reef, Queensland, and from the stout longtom, Tylosurus gavialoides, from Moreton Bay, Queensland. In this study, novel SEM data of specimens of Sch. huffmani are presented to compare with samples obtained from the EP and GoM. Australian specimens for SEM were transferred from ethanol to hexamethyldisilazane, air-dried overnight and mounted on 12.5 mm pin-stubs using an adhesive carbon tab. Before performing SEM, specimens were coated with 15 nm of iridium with a Quorumtech Q150TS sputter coater. SEM images were obtained on a Hitachi SU3500 scanning electron microscope in secondary electron mode.
DNA sequencing and phylogenetic analyses
Specimens preserved in 100% EtOH were digested overnight at 56°C in a solution containing 10 mm Tris-hydrochloric acid (pH 7.6), 20 mm sodium chloride, 100 mm Na2EDTA (pH 8.0), 1% sarkosyl and 0.1 mg mL−1 proteinase K. DNA was isolated from the supernatant using DNAzol (Molecular Research Center, Cincinnati, OH, USA) following the manufacturer's instructions. Fragments of the large (28S) and small (18S) subunits of ribosomal DNA were amplified via polymerase chain reaction using MyTaq™ DNA polymerase and the primers 502 (5′-CAA GTA CCG TGA GGG AAA GTT GC-3′) and 536 (5′-CAG CTA TCC TGA GGG AAA C-3′) (García-Varela and Nadler, Reference García-Varela and Nadler2005), and G18S4 (5′-GCT TGT CTC AAA GAT TAA GCC-3′) and 136 (5′-TGA TCC TTC TGC AGG TTC ACC TAC-3′) (Choudhury and Nadler, Reference Choudhury and Nadler2018), respectively. A fragment of the mitochondrial gene cytochrome oxidase C subunit 1 (cox1) was amplified with the primers Dig_CoxFa (5′-ATG ATW TTY TTY TTY YTD ATG CC-3′) and Dig_CoxR (5′-TCN GGR TGH CCR AAR AAY CAA AA-3′) (Wee et al., Reference Wee, Cribb, Bray and Cutmore2017). Contiguous sequences were assembled, and base-calling differences were resolved, using Geneious Pro 4.8.4 (Biomatters Ltd., Boston, USA). Sequences were deposited in the GenBank database.
DNA sequences were aligned using MUSCLE (Edgar, Reference Edgar2004) through the EMBL-EBI web interface (Madeira et al., Reference Madeira, Park, Lee, Buso, Tamer, Madhusoodanan, Basutkar, Tivey, Potter, Finn and Lopez2019). Additional sequences of representatives of plagiorchiidans of the families Notocotylidae (Catatropis indicus Srivastava, 1935), Opisthotrematidae (Lankatrema mannarense Crusz & Fernand, 1954), Diplodiscidae (Diplodiscus subclavatus (Goeze, 1782)), Cladorchiidae (Solenorchis travassosi Hilmy, 1949), Psilostomidae (Neopsilotrema lakotae Kudlai, Pulis, Kostadinova & Tkach, 2016 and Psilochasmus oxyurus (Creplin, 1851) [Lühe, 1909]) and Echinostomatidae (Echinostoma revolutum (Fröhlich, 1802) Looss, 1899) were incorporated as outgroup taxa (Table 1). A substitution model was inferred using MrModeltest v. 2.3 (Nylander, Reference Nylander2004) following the Akaike's information criterion, obtaining GTR + I + G as the best model. Bayesian inference (BI) analyses were performed independently for each of the 3 genes. The 2 ribosomal genes were then concatenated, and a BI analysis was conducted to infer the interrelationships among the specimens sampled and other haplosplanchnid species available in GenBank. BI analysis was conducted using MrBayes v. 3.2.2 (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Hohna, Larget, Liu, Suchard and Huelsenbeck2012) on the CIPRES Science Gateway (Miller et al., Reference Miller, Pfeiffer and Schwartz2010). The analysis included 2 simultaneous runs of Markov chain Monte Carlo, each for 4 million generations, sampling trees every 4000 generations, a heating parameter value of 0.2 and a ‘burn-in’ of 25%. A 50% majority-rule consensus tree was constructed from the post burn-in trees. BI outputs were imported to FigTree v. 1.4 (Rambaut, Reference Rambaut2014) for graphical visualization and editing.
Table 1. GenBank accession numbers of DNA sequences used in phylogenetic analyses of members of the order Haplosplanchnata during this study

A neighbour-joining (NJ) tree was generated in MEGA v. 11 (Tamura et al., Reference Tamura, Stecher and Kumar2021) using the cox1 sequences of Schikhobalotrema species from Australia and Mexico, based on the Tamura–Nei model, gamma distribution rate and 500 bootstrap replicates. Genetic divergence (P-distance and number of nucleotide differences) between the new species and other haplosplanchnids was also calculated in MEGA.
Results
General phylogenetic results
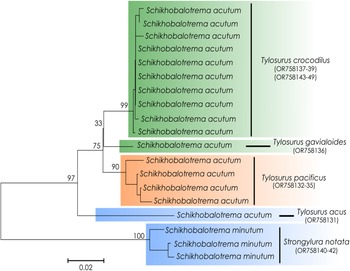
Specimens from the intestines of needlefishes in 2 localities of the Pacific coast of Mexico and 2 localities off Yucatán in the GoM were initially morphologically identified as Schikhobalotrema acutum. Four specimens obtained from Strongylura spp., 1 specimen from T. acus and 4 specimens from T. pacificus were sequenced for all 3 molecular markers; the 28S alignment was 1094 bp, the 18S alignment was 1732 bp and the cox1 alignment was 475 bp. In the phylogenetic tree inferred with the concatenated dataset (18S + 28S), the family Haplosplanchnidae resolved as a monophyletic group, as did the genus Schikhobalotrema (Fig. 1); in both cases, relationships were strongly supported.

Figure 1. Relationships between members of the family Haplosplanchnidae inferred from BI analysis of the concatenated dataset (18S + 28S). Schikhobalotrema species along with host and sample site are also shown in the tree. Values at the nodes indicate posterior probabilities. GenBank accession numbers are shown in Table 1. Scale bar = number of substitutions per site.
The type-species of the genus, Sch. acutum, collected from T. acus from the GoM and T. pacificus from off Chamela Bay and Barra de Coyuca, Acapulco on the Mexican Pacific coast formed a strongly supported clade with sequences of Sch. huffmani collected from T. crocodilus and T. gavialoides from Australia (Fig. 1). The genetic divergence values for the 2 ribosomal genes between Sch. huffmani from Australia and Sch. acutum from the EP and GoM were very low, just 1–5 base positions for 28S (0.1–0.3%) and 1–2 base positions for 18S (0.1%). The cox1 divergence among isolates of Schikhobalotrema occurring in species of Tylosurus across the same geographic range was 3.2–7.4% (15–32 base positions). Given the similarities in morphology, host and molecular data, we here consider Sch. huffmani a junior synonym of Sch. acutum (see Remarks section for Sch. acutum).
In both the 18S + 28S concatenated analysis and the cox1 analysis, Sch. acutum resolved as sister to a highly supported and reciprocally monophyletic clade comprising specimens of Schikhobalotrema collected from 2 species of Strongylura in La Carbonera coastal lagoon (Figs 1 and 2). This clade differed from Sch. acutum by 1–1.6% (8–13 base positions) in the 28S data, 0.1–0.2% (3–5 base positions) in the 18S data and 12.5–14.5% (56–65 base positions) in the cox1 data. Given the distinction in genetic data in sympatry (particularly the cox1 data), we consider this clade to represent a distinct species, which we describe as Schikhobalotrema minutum n. sp.

Figure 2. Phylogram of the NJ analysis of cox1 for species of Schikhobalotrema. Values at the nodes indicate posterior probabilities. GenBank accession numbers included after the species name. Scale bar = number of substitutions per site. Green colour refers to Australia; orange refers to the Pacific coast of Mexico; blue refers to GoM.
Family Haplosplanchnidae Poche, 1926
Genus Schikhobalotrema Skrjabin & Guschanskaja, 1955
Schikhobalotrema acutum (Linton, Reference Linton1910) Skrjabin & Guschanskaja, 1955 (Figs 3B, C; 4B, C, D; 5B, C, D; 6B, C, D).
Synonyms: Distomum sp. of Linton (Reference Linton1907); Deradena acuta Linton, Reference Linton1910; Haplosplanchnus acutus (Linton, Reference Linton1910) Manter, Reference Manter, Schulz and Gnyedina1937; Schikhobalotrema huffmani Huston, Cutmore & Cribb, Reference Huston, Cutmore and Cribb2017 (see Table 2).

Figure 3. Line drawings of species of Schikhobalotrema from marine and estuarine fishes of Mexico: (A) Schikhobalotrema minutum n. sp. ex Strongylura notata, ventral view; (B) Schikhobalotrema acutum ex Tylosurus acus, ventral view and (C) Sch. acutum ex Tylosurus pacificus, lateral view. Scale bars = 500 μm.

Figure 4. SEM photomicrographs of the entire body of 3 species of Schikhobalotrema: (A) Sch. minutum n. sp. ex St. notata, La Carbonera coastal lagoon, Yucatán, Mexico; (B) Sch. acutum ex T. acus, off Celestún, Yucatán, Mexico; (C) Sch. acutum ex T. pacificus, off Barra de Coyuca, Acapulco, Mexico and (D) Sch. acutum ex Tylosurus crocodilus, Lizard Island, Great Barrier Reef, Australia. Scale bars = 200 μm.

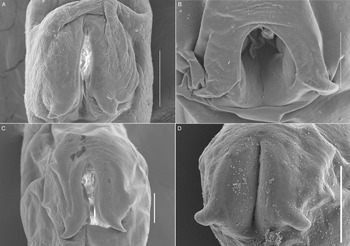
Figure 5. SEM photomicrographs of the oral sucker of 3 species of Schikhobalotrema showing the distribution of papillae: (A) Sch. minutum n. sp. ex St. notata, La Carbonera coastal lagoon, Yucatán, Mexico; (B) Sch. acutum ex T. acus, off Celestún, Yucatán, Mexico; (C) Sch. acutum ex T. pacificus, off Barra de Coyuca, Acapulco, Mexico and (D) Sch. acutum ex T. crocodilus, Lizard Island, Great Barrier Reef, Australia. Scale bars = 50 μm.

Figure 6. SEM photomicrographs of the ventral sucker of 3 species of Schikhobalotrema showing 2 lateral appendages on the posterior end: (A) Sch. minutum n. sp. ex St. notata, La Carbonera coastal lagoon, Yucatán, Mexico; (B) Sch. acutum ex T. acus, off Celestún, Yucatán, Mexico; (C) Sch. acutum ex T. pacificus, off Barra de Coyuca, Acapulco, Mexico and (D) Sch. acutum ex T. crocodilus, Lizard Island, Great Barrier Reef, Australia. Scale bars = 100 μm.
Table 2. Measurements of some morphological traits of Schikhobalotrema species parasitizing belonids

B, Body; OS, oral sucker; VS, ventral sucker; PF, pre-pharynx; PH, pharynx; OE, oesophagus; C, caecum; T, testis; OV, ovary, E, egg.
Mean value in bold.
a Flattened specimens.
Type-host: Strongylura marina (Walbaum) (Beloniformes: Belonidae).
Other hosts: See Table 3.
Table 3. Reports of Sch. acutum

D, description; F, figures; M, molecular data.
All host names updated to current interpretations. See Discussion for comments.
Type-locality: Dry Tortugas, Florida, Gulf of Mexico.
Other localities: See Table 3.
New material examined: 10 vouchers ex T. pacificus from off Chamela Bay, CNHE 12035; 6 vouchers ex T. pacificus from off Barra de Coyuca, Acapulco, CNHE 12036 and 1 voucher ex T. acus from off Celestún, Yucatán, CNHE 12034.
Site in host: Intestine.
Prevalence of infection: Three of 10 T. pacificus, Chamela Bay, Jalisco, Mexico; 4 of 6 T. pacificus, Barra de Coyuca, Acapulco, Guerrero, Mexico; 1 of 3 T. acus, Celestún, Yucatán, Mexico.
Representative DNA sequences: ex T. pacificus, 4 sequences from off Barra de Coyuca, Acapulco, Guerrero: 28S rDNA (OR753902–OR753904), 18S rDNA (OR753906) and cox1 (OR758132–OR758135); ex T. acus from off Celestún, Yucatán, 1 sequence: 28S rDNA (OR753897), 18S rDNA (OR753905) and cox1 (OR758131); ex T. gavialoides, 1 sequence from Moreton Bay: cox1 (OR758136); ex T. crocodilus, 10 sequences from off Lizard Island (OR758137–OR758139, OR758143–OR758149).
Description: Measurements are provided in Table 2. Our specimens were consistent with previous descriptions of this species by other authors (e.g. Linton, Reference Linton1910; Manter, Reference Manter, Schulz and Gnyedina1937, Caballero et al., Reference Caballero, Bravo-Hollis and Grocott1953, Huston et al., Reference Huston, Cutmore and Cribb2017).
Remarks: We were unable to detect any qualitative morphological or morphometric differences between our specimens of this species from Mexico and those of Sch. huffmani from Australia. Additionally, both phylogenetic analyses resolve the Australian specimens of Sch. huffmani and specimens of Sch. acutum from the Pacific coast of Mexico as sister clades with high nodal support (Figs 1 and 2). The level of genetic difference between the Australian and Mexican specimens is relatively low; specimens from Australia differ from those from the Pacific coast of Mexico at just 13–21 base positions (3.1–4.6%) and from those from the GoM at 30–32 base positions (7.2–7.6%) in the cox1 dataset, which is the most variable marker analysed. Although Huston et al. (Reference Huston, Cutmore and Cribb2017) noted morphological differences between their specimens and those of Sch. acutum from other studies, inclusion of the new specimens somewhat erodes these distinctions. Huston et al. (Reference Huston, Cutmore and Cribb2017) noted that Sch. huffmani had a smaller body and thus smaller general features; however, some of the new material from Mexico studied here is much closer in size to those of Sch. huffmani than from other reports of Sch. acutum. Other morphological differences reported by Huston et al. (Reference Huston, Cutmore and Cribb2017) between their specimens and Sch. acutum were based mainly on the description given by Manter (Reference Manter, Schulz and Gnyedina1937); most other descriptions of this species have largely lacked in finer detail. It seems likely that these differences would have been considered less significant if specimens from other previous studies were considered. Considering the evidence available (morphological, molecular and host specificity), we conclude that Sch. huffmani is best considered a synonym of Sch. acutum.
Schikhobalotrema minutum n. sp. (Figs 3A, 4A, 5A, 6A).
Type-host: Strongylura notata (Beloniformes: Belonidae).
Other hosts: Strongylura marina (Beloniformes: Belonidae).
Type-locality: La Carbonera coastal lagoon (21°13′48.2″ N; 89°53′20.5″ W).
Type-material: Holotype (CNHE 12032) and 5 paratypes (CNHE 12033).
Site in host: Intestine.
Prevalence of infection: Three of 24 St. notata (12.5%); 1 of 2 St. marina (50%).
Representative DNA sequences: ex St. notata 3 sequences: 28S rDNA (OR753898–OR753899, OR753901), 18S rDNA (OR753907–OR753908, OR753910) and cox1 (OR758140–OR758142). ex St. marina, 1 replicate for each gene: 28S rDNA (OR753900) and 18S rDNA (OR753909).
Etymology: The specific epithet refers to the overall smaller body size of the new species compared with the congeneric species for which sequence data are available.
ZooBank LSID: urn:lsid:zoobank.org:pub:9982DE1C-90C5-45DF-B608-04AA79609C5F.
Description (Based on 6 dorso-ventrally mounted specimens, 5 ex St. notata and 1 ex St. marina from La Carbonera coastal lagoon and 1 specimen processed for SEM). Body small, tapering anteriorly and posteriorly, 378–761 × 164–316 (545 × 223) (Figs 3A and 4A). Tegument thin, lacking dorsal annulations. Eyespot pigment dispersed in forebody, around pharynx. Oral sucker round, 76–134 × 82–134 (96 × 108), bearing conspicuous frontal gland with a pore in ventral lip and 6 pairs of papillae arranged symmetrically (Fig. 5A). Ventral sucker large, larger than oral sucker, in midbody, with 2 conspicuous lateral appendages at posterior end, 131–207 × 94–197 (165 × 141) not considering lobe extensions; aperture a longitudinal slit (Figs 3A, 4A and 6A). Ventral sucker/oral sucker length ratio 1.3–2.2 (1.7):1; ventral sucker/oral sucker width ratio 1.1–1.5 (1.3):1. Forebody 115–240 (175), occupying 30–33 (32%) of body length. Prepharynx distinct, 23–70 (41). Pharynx ovoid, 47–74 × 48–66 (63 × 53). Oesophagus inconspicuous, 26–41 (35) long. Caecum extending dorsally to level with anterior margin of ovary.
Testis singular, in mid-posterior third of body, transversally elongate, 80–185 × 60–140 (130 × 90), occupying 24–25 (25%) of body length. Seminal vesicle short, tubular, forming a globular prostatic bulb before opening at genital atrium. Genital pore ventral, lateral to pharynx. Ovary irregularly shaped, slightly overlapping anterior portion of testis, 44–86 × 33–84 (70 × 60). Laurer's canal and Mehlis gland not observed. Seminal receptacle globular, lateral to ovary. Vitellarium follicular, in 2 lateral fields extending from anterior margin of ventral sucker to mid-region between posterior margin of testis and posterior extremity; fields apparently not confluent. Uterus passes ventral sucker dorsally. Eggs large, 61–90 × 38–57 (73 × 50). Excretory vesicle I-shaped. Excretory pore terminal.
Remarks: In the possession of a ventral sucker with 1 pair of lateral appendages on the posterior margin and a longitudinal aperture, the new species is similar to 3 species of Schikhobalotrema: Sch. acutum, Schikhobalotrema ablennis (Abdul-Salam and Khalil, Reference Abdul-Salam and Khalil1987) Madhavi, Reference Madhavi, Jones, Bray and Gibson2005 and Schikhobalotrema adacutum (Manter, Reference Manter, Schulz and Gnyedina1937) Skrjabin and Guschanskaja, 1955. The new species differs from Sch. ablennis in having a more anterior testis and a shorter forebody. Further, Sch. ablennis was reported from a different genus of belonid Ablennes Jordan & Fordice (see Abdul-Salam and Khalil, Reference Abdul-Salam and Khalil1987), whereas the new species was found in Strongylura spp. The new species differs from Sch. adacutum in having a more conspicuous lateral appendage of the ventral sucker, and even though both are found in the GoM, Sch. adacutum is known from species in the families Atherinidae, Hemiramphidae, Labridae, Scaridae and Pomacentridae, and not members of the Belonidae.
Morphologically the new species most closely resembles Sch. acutum which is also reported from belonids. These 2 species share the presence of a conspicuous frontal gland in the ventral lip of the oral sucker but are distinct in that there are 6 pairs of papillae symmetrically arranged around the oral sucker in the new species, vs 7–8 pairs for Sch. acutum (Fig. 5). The new species further differs from Sch. acutum by the possession of a caecum that extends to level with the ovary, vs to level with the middle portion of the testis in Sch. acutum (Fig. 3). Further, the new species differs in the overall body size (it is distinctly smaller), and thus possesses distinctly smaller oral and ventral suckers, pharynx and genitalia (Table 2, Fig. 3). Finally, the new species is clearly genetically distinct from Sch. acutum for all 3 gene regions analysed in this study.
Discussion
Trematode identification over geographical range
Recently there have been numerous studies using cox1 data to explore the delimitation of marine fish trematodes over wide geographic ranges in the IWP. Huston et al. (Reference Huston, Cutmore, Miller, Sasal, Smit and Cribb2021) tested species boundaries within the gorgocephalid genus Gorgocephalus Manter, 1966 in the IWP, demonstrating that 3 of the 4 Gorgocephalus species studied had convincingly wide distributions in the region, with Gorgocephalus yaaji distributed across the IWP extremes (South Africa to French Polynesia). Cutmore et al. (Reference Cutmore, Yong, Reimer, Shirakashi, Nolan and Cribb2021) reported that some species of blood flukes (Aporocotylidae) are widely distributed in the IWP, with cox1 data indicating that Ankistromeces olsoni Nolan & Cribb, 2006 is found from Australia to Japan and Phthinomita sasali Nolan & Cribb, 2006 is found from Ningaloo Reef in the Indian Ocean to Palau in the Pacific Ocean. Cutmore and Cribb (Reference Cutmore and Cribb2022) demonstrated that the blood fluke Elaphrobates chaetodontis (Yamaguti, 1970) Yong, Cribb & Cutmore, 2021 is similarly widespread, from cox1 data from Australia, Japan and French Polynesia forming a well-supported and geographically structured clade. Wee et al. (Reference Wee, Cutmore and Cribb2022) demonstrated that Helicometroides longicollis Yamaguti, 1934 (Monorchiidae) is distributed between Japan and Australia, and Cribb et al. (Reference Cribb, Bray, Justin, Reimer, Sasal, Shirakasi and Cutmore2022) showed that Bivesicula claviformis Yamaguti, 1934 is found, at least, in Japan and of both the Indian and Pacific coasts of Australia. It must be noted, however, that for each of these widespread species, all these above studies demonstrated that at least some of their congeners were highly restricted with equally convincing data.
Most notably, Bray et al. (Reference Bray, Cutmore and Cribb2022) studied species of the lepocreadiid genus Preptetos Pritchard, 1960 infecting acanthurid fishes from a range of sites in the IWP. In this study, the authors proposed a set of objective criteria for the recognition of trematode species, with as a first step the reciprocal monophyly using the most discriminating available molecular marker, and at least one of the following criteria: consistent morphological differences relative to other species, or consistent differences in host distribution with respect to close relatives. In our study, we followed the paradigm proposed by Bray et al. (Reference Bray, Cutmore and Cribb2022) analysing the morphological and molecular variation, host association (host-specificity), the historical biogeography of the host group, the habitat of the host and the geographical distribution of both associates to determine whether the distinct genetic lineages correspond to separate species of Schikhobalotrema.
The new species, Sch. minutum n. sp. was resolved as a reciprocally monophyletic group in both the 18S + 28S rDNA and cox1 mtDNA phylogenetic analyses (Figs 1 and 2). The new species differed from Sch. acutum at 56–65 base positions (12.5–14.5%) in the cox1 dataset, a difference consistent with the recognition of closely related but distinct species for a range of trematode families in the IWP (e.g. Cutmore et al., Reference Cutmore, Yong, Reimer, Shirakashi, Nolan and Cribb2021, Reference Cutmore, Corner and Cribb2023; Huston et al., Reference Huston, Cutmore, Miller, Sasal, Smit and Cribb2021; Bray et al., Reference Bray, Cutmore and Cribb2023; Magro et al., Reference Magro, Cutmore, Carrasson and Cribb2023). Further, for Sch. minutum n. sp. we observed some morphological characters to differentiate the new species, mostly based on body size and the extension of the caecum in the body. Relative to the paradigm proposed by Bray et al. (Reference Bray, Cutmore and Cribb2022), these traits support the recognition of a new species.
Interpretation of the new specimens relating to Sch. acutum relative to the description of Sch. huffmani is more complex, with reports from across the Pacific and Atlantic oceans. Schikhobalotrema acutum was originally described from the GoM, but it has since been reported from the Caribbean Sea, Brazil, Colombia, the Galapagos Islands, Panama, India, Japan and the Philippines (Manter, Reference Manter1940; Caballero et al., Reference Caballero, Bravo-Hollis and Grocott1953; Siddiqi and Cable, Reference Siddiqi and Cable1960; Madhavi, Reference Madhavi1979; Machida and Kuramochi, Reference Machida and Kuramochi2000; Kohn et al., Reference Kohn, Fernández and Cohen and2007). Although molecular data are not available for samples from most of the host records, we now have cox1 data for samples from 4 marine realms, the tropical Atlantic, tropical EP, the central Indo-Pacific and temperate Australia (see Spalding et al., Reference Spalding, Fox, Halpern, McManus, Molnar, Allen, Davidson, Jorge, Lombana, Lourie, Martin, McManus, Recchia and Robertson2007). These data demonstrate that populations of Sch. acutum across a wide geographic range have only small cox1 divergence values; new samples from the GoM and the Pacific coast of Mexico differ at just 30–34 base positions (7.2–7.9%), and those from the Pacific coast of Mexico and Australia differ at just 13–21 base positions (3.1–4.6%). These values, and the lack of divergence for both ribosomal genes, support the interpretation of a single species across these regions. These divergence values generally agree with those obtained for other marine fish trematode genera. McNamara et al. (Reference McNamara, Miller and Cribb2014) tested the identity of 16 morphospecies of Hurleytrematoides Yamaguti, 1954 parasitizing a wide range of Chaetodontidae species in the IWP. They recognized species boundaries at a minimum of 55 base positions (9.1%) in cox1. Confidence in these interpretations was supported by the fact that the morphospecies of Hurleytrematoides show clear distinctions in their complex terminal genitalia, contrasting with the general morphological similarity of many combinations of haplosplanchnid species. Increasingly, however, studies are demonstrating the propensity for cryptic speciation in the Trematoda which seems to be a pattern as suggested by Pérez-Ponce de León and Poulin (Reference Pérez-Ponce de León and Poulin2018), and morphological differences supporting genotypic distinctions are not always evident. As for the studies by Huston et al. (Reference Huston, Cutmore, Miller, Sasal, Smit and Cribb2021), Bray et al. (Reference Bray, Cutmore and Cribb2022), Cribb et al. (Reference Cribb, Bray, Justin, Reimer, Sasal, Shirakasi and Cutmore2022) and Cutmore et al. (Reference Cutmore, Yong, Reimer, Shirakashi, Nolan and Cribb2021), the hypothesis we present herein for Schikhobalotrema is based heavily on the interpretation of the cox1 data; although partially supportive of the cox1 relationships, ribosomal data are proving to be less informative to delineate species in trematodes that have conserved morphology, but still a proper marker to separate species (see Pérez-Ponce de León and Hernández-Mena, Reference Pérez-Ponce de León and Hernández-Mena2019). Genetic data are not available for most of the species in the genus Schikhobalotrema; sequence data have been generated for just 4 of the 27 valid species. Clearly, genetic information for more congeneric species is needed to achieve robust conclusions regarding patterns of geographical distribution and host-specificity in this trematode genus. DNA information, particularly that from the most variable marker, the mitochondrial cox1, proved critical for drawing conclusions in the current study.
Biogeography
In this study, we provide molecular and morphological data in support of the hypothesis that Sch. acutum is distributed across a wide geographic range incorporating the GoM, the western Pacific and the EP. Some marine organisms, particularly certain fishes, can readily disperse over great distances. For instance, it has been shown that some species may pass what Lessios and Robertson (Reference Lessios and Robertson2006) considered the ‘impassable’ eastern Pacific barrier, ca. 5000 km of deep water that separates the eastern from the central Pacific which is the widest marine biogeographic barrier in the world (Lessios and Robertson, Reference Lessios and Robertson2006). While there have been no molecular studies published regarding broad distributions of the belonid genus Tylosurus, some species are known to have wide ranges; according to FishBase the hound needlefish, T. crocodilus, is distributed in the IWP, the tropical Atlantic, and off the coasts of Africa and the Americas. Wide host ranges of fishes are, however, not necessarily mirrored by their trematode parasites; due to the complex life cycles of trematodes and the absence of long-lived dispersive larval stages, they may have far more restricted ranges. There are, however, some notable exceptions for those species infecting highly vagile marine hosts, such as the blood flukes infecting bluefin tuna (Aiken et al., Reference Aiken, Bott, Mladineo, Montero, Nowak and Hayward2007) and spirorchiid blood flukes of marine turtles (Corner et al., Reference Corner, Cribb and Cutmore2022, Reference Corner, Cribb and Cutmore2023). Our current interpretation of the available sequence data for specimens morphologically consistent with Sch. acutum indicates that it has an exceptional distribution, occurring from Australia to the Mexican Pacific coast and the GoM. Molecular characterization of samples from other reported localities and other reported belonid hosts (Tylosurus fodiator, Strongylura incisa, St. marina and Strongylura timucu) will doubtless further improve understanding of this system.
While it appears that a close phylogenetic relationship between hosts and parasites, and some life-history traits of the host, seem to explain the large geographic range exhibited by Sch. acutum, more sequence data and the analysis of other trematode species and even other parasite taxa such as monogeneans and copepods (which also seem to exhibit a strong host-specificity towards belonids) will prove useful for understanding the historical biogeography of belonids and their parasitic fauna.
Host-specificity
Table 3 summarizes host reports of Sch. acutum, including the 3 new records made here and interpreting the original reports of Sch. huffmani as Sch. acutum. Multiple higher taxa of fishes are involved, but the Beloniformes account for well over half the reports. In our view, none of the reports from other orders of fishes is strongly credible given the general lack of evidence provided and the rarity of the combinations: the single record from an atherinopsid by Sogandares-Bernal (Reference Sogandares-Bernal1959) was considered ‘accidental’ in the original report and lacked evidence; the report from a kyphosid was of a single specimen (Manter, Reference Manter1940) and lacked evidence; the report from a labrid was of 11 individual worms (Fischthal, Reference Fischthal1977) but lacked evidence; the report from a lutjanid was of 3 specimens (Fischthal and Nasir, Reference Fischthal and Nasir1974) but lacked evidence; the report from a mugilid by Fayek et al. (Reference Fayek, Amer and Ahmed1990) can be unambiguously discounted on the basis that the ventral sucker is shown as lacking processes and the eggs are embryonated with miracidia; the report from pomacentrid has an image consistent with Sch. acutum but was of a non-gravid specimen (Sogandares-Bernal and Sogandares, Reference Sogandares-Bernal and Sogandares1961) and the report from trichiurid was of 2 worms (Fischthal and Nasir, Reference Fischthal and Nasir1974) but lacked evidence. It seems unlikely that any of these fishes represent regular hosts for Sch. acutum and certainly all need further verification.
Infections of Sch. acutum are clearly concentrated in beloniforms and, among them, in belonids (although many of these reports also lack evidence). However, 4 studies have reported infections from hemiramphids. Cable (Reference Cable1954), in work directed at the first elucidation of a haplosplanchnid life cycle, reported that Sch. acutum was ‘common in needle-fish and half-beaks’ in his study area. Siddiqi and Cable (Reference Siddiqi and Cable1960) reported Sch. acutum from both a belonid and a hemiramphid but without any prevalence details. Madhavi (Reference Madhavi1979) reported Sch. acutum from both a belonid and 2 hemiramphids but did not report prevalence data and her figure did not indicate the host of the sample. Machida and Kuramochi (Reference Machida and Kuramochi2000) reported a single specimen of Sch. acutum from a hemiramphid together with 4 from belonids; their descriptions did not distinguish between specimens from the 2 families. Given the expertise of the workers involved the reports seem broadly credible, but we consider the issue of whether Sch. acutum is genuinely shared by both belonids and hemiramphids to be unresolved. In favour of the sharing are the repeated reports by multiple experts together with the fact that the 2 fish families belong to the same order of fishes and occupy similar habitats. Against the sharing is the lack of positive evidence (descriptions, figures, molecular data) of the infections in hemiramphids and the fact that, although related, belonids and hemiramphids have dramatically differing diets. Belonids are overwhelmingly piscivores whereas hemiramphids are omnivorous, eating mainly algae and invertebrates. In this context we note that we have examined 237 hemiramphid individuals from Australian localities where infection in belonids were detected but have found no infections of Sch. acutum; a similar situation holds true for Mexican localities of both the Pacific and the GoM coasts where we have examined around 130 hemiramphids, in which Sch. acutum has not been found.
The presence of haplosplanchnids in belonids is essentially unexplained. What is known of haplosplanchnid life cycles suggests that typically their cercariae encyst in the open (Cable, Reference Cable1954; Fares and Maillard, Reference Fares and Maillard1975) probably typically in association with algae. This form of transmission is consistent with their concentration in herbivorous fishes. According to our records, just 4 fish families (Acanthuridae, Labridae [overwhelmingly the subfamily Scarinae], Mugilidae and Pomacentridae), which all incorporate significant grazing of algae, account for over 80% of the host records for haplosplanchnids. No family of piscivores other than the Belonidae is significant as hosts. Given the presence of species of Schikhobalotrema with paired appendages on the ventral sucker in both belonids and hemiramphids, we predict that infections in belonids arose as a host switch into the latter family. However, the mode of transmission remains unknown.
Data availability statement
Sequence data are available in the NCBI GenBank database.
Acknowledgements
We sincerely thank Laura Márquez and Nelly López from the Laboratorio Nacional de Biodiversidad (LaNabio, Biology Institute, UNAM) for their help with DNA sequencing processing and Maribel Badillo Alemán and Alfredo Gallardo from BioCon (Laboratorio de Biología de la Conservación, F.C., UNAM) for technical support, sampling of fish in Yucatán and host identification.
Author's contribution
G. P.-P. L. and B. S.-G. designed the study. G. P.-P. L., B. S.-G., J. C.-G. and B. M.-G. conducted fieldwork, processed the specimens from Mexico for morphological and molecular analyses and drew the morphological figures. D. C. H., T. H. C. and S. C. C. processed the specimens from Australia and obtained cox1 sequences. B. S.-G. analysed the molecular data and submitted the sequences to GenBank. G. P.-P. L. and T. H. C. discussed the results. G. P.-P. L. drafted the manuscript which was reviewed and greatly improved by all authors.
Financial support
This research was supported by grants from the Programa de Apoyo a Proyectos de Investigación e Inovación Tecnológica (PAPIIT-UNAM IN212621), and the Consejo Nacional de Ciencia y Tecnología (CONACYT A1-S-21694) to G. P.-P. L. S. C. C. is supported by an Australian Biological Resources Study National Taxonomy Research Grant (4-H04JDSM).
Competing interests
None.
Ethical standards
All applicable institutional, national and international guidelines for the care and use of animals were followed. Fish analysed in this study were collected from commercial capture.