Introduction
Cardiovascular disease (CVD) is the most common non-communicable disease occurring globally and is responsible for about 18·6 million deaths every year(Reference Roth, Mensah and Johnson1), significantly impacting individuals who are in the peak productive period of life and consequentially placing an economic burden of billions of dollars on health care systems and the wider society(Reference Bloom, Cafiero and Jané-Llopis2). Reducing premature mortality from non-communicable diseases, including CVD, by one-third is a global priority put forth as part of the United Nations (UN) Sustainable Development Goals(3). Better identification and management of CVD-related risk factors could prevent thousands of premature deaths that result from CVD(4).
Diet is one factor that plays an important role in CVD prevention and treatment. Understanding the causal role of diets in the prevention and treatment of CVD would traditionally require randomised controlled trials (RCTs), as they are often considered the gold standard in establishing causal relationships. However, RCTs are not always the most appropriate or feasible design in nutrition research, particularly for CVD. For instance, RCTs can be costly as it can take several years before CVD outcomes can be directly measured. Observational prospective studies, which are the second option in terms of causality, are not without limitations. They are often criticised because unmeasured factors that influence both exposures and outcomes might be responsible for the relationship reported in studies. In addition, controlling for variables that mediate the relationship between exposures and outcomes can introduce errors in the estimation of effects(Reference VanderWeele5). Causal diagrams used in causal inference analysis can be a useful tool to improve decisions with regard to selection and controlling for variables in observational studies and avoid potential biases introduced by purely data-driven methods that do not differentiate confounders and mediators(Reference Shrier and Platt6). Expert knowledge regarding the causal structure of the relationship among diet and physiological risk factors for CVD is warranted.
In this narrative review, we aim to present diagrams illustrating current evidence on the links between diet, as well as conventional and emerging physiological risk factors, and CVD risk, incidence and mortality. To facilitate the visualisation of potential causal links (i.e. where randomised studies exist) as well as areas for further research, the diagrams display information regarding types of studies. The diagrams for conventional and emerging physiological risk factors for CVD was informed by studies included in the British Nutrition Task Force report on diet and CVD, which was published in 2019. This report comprehensively reviewed and summarised the state of knowledge on major emerging risk factors for CVD(Reference Stanner, Stanner, Coe, Frayn and Keith7). In addition to extracting key primary and secondary references from this authoritative report, database searches across Medline and Embase were conducted to identify additional studies that might not have been captured by the aforementioned report. Searches were limited to systematic reviews published from 2018 onwards (for search strategy, see Appendix A). The diagrams depicting the role of diet in the aetiology of CVD was also informed by the British Nutrition Task Force report and supplemented by a recent umbrella review(Reference Chareonrungrueangchai, Wongkawinwoot, Anothaisintawee and Reutrakul8) and additional relevant studies identified using a key terms approach. This narrative review aims to present a comprehensive but not necessarily exhaustive view of conventional and emerging risk factors for CVD. The second aim of this paper is to discuss the potential application of the diagrams to studies using tools from causal inference.
Conventional risk factors for CVD
Hypertension and blood pressure disturbances
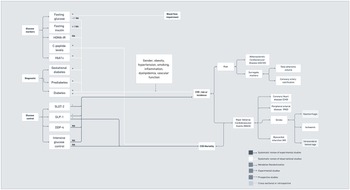
Figure 1 depicts a summary of the relationships linking markers of hypertension and blood pressure disturbances with CVD outcomes. In terms of observational studies, recent evidence has suggested significant associations between different alterations in blood pressure with increased cerebro- and cardiovascular risk and events, including increased systolic blood pressure(Reference Pan, Hibino, Kobeissi and Aune9), central systolic blood pressure(Reference Li, Huang and Feng10) and systolic blood pressure variability(Reference Appiah, Patel, Panerai, Robinson and Haunton11–Reference Ma, Song and Viswanathan15), exaggerated systolic hypertensive response to exercise(Reference Perçuku, Bajraktari, Jashari, Bytyçi, Ibrahimi and Henein16) and orthostatic hypotension(Reference Min, Shi and Sun17). Patients with masked hypertension(Reference Zhang, Guo, An, Li and Wang18,Reference Pierdomenico, Pierdomenico and Coccina19) and white coat hypertension(Reference Cohen, Lotito, Trivedi, Denker, Cohen and Townsend20,Reference Fujiwara, Matsumoto, Asayama, Ohkubo and Hoshide21) have also shown increased CVD risk when compared with normotensive patients. One large prospective study in the UK also showed that sustained hypertension has stronger associations with CV events when compared with single blood pressure measurements, but inclusion of long-term systolic blood pressure did not necessarily translate to better performance of CVD prediction tools(Reference Ayala Solares, Canoy and Raimondi22). The presence of hypertensive disorders during pregnancy(Reference Kirollos, Skilton, Patel and Arnott23) has also been associated with increased changes in the vascular structure of the mother, which might contribute to increased CVD risk. Despite the variety of markers considered in systematic reviews of observational studies, only a few markers were explored for their association with CVD mortality. In addition, conclusions drawn from observational studies were often limited by the low number of studies available for some markers (e.g. blood pressure variability and masked hypertension), the tendency toward publication bias, heterogeneity across studies, which hinders comparability, and the lack of proper adjustment for confounders.

Fig. 1. Hypertension and blood pressure disturbances associated with CVD risk, incidence and mortality.
In terms of randomised controlled trials (RCTs), recent reviews have shown that, compared with standard goals, intensive systolic or blood pressure reduction was accompanied by larger reductions in stroke, coronary heart disease(Reference Salam, Atkins and Sundström24), myocardial infarction and major adverse cardiovascular events(Reference Sakima, Satonaka, Nishida, Yatsu and Arima25,Reference Fei, Tsoi and Cheung26) . Intensive reductions in diastolic blood pressure were further associated with reduced CVD mortality(Reference Fei, Tsoi and Cheung26). However, the benefits of intensive reductions in blood pressure were heterogeneous across different groups. For instance, among patients with type 2 diabetes, data show a linear relationship between systolic blood pressure reduction and the occurrence of stroke(Reference Saiz, Gorricho, Garjón, Celaya, Erviti and Leache27), but not other CVD events or mortality. Among hypertensive patients with a history of coronary artery disease, lower targets reduced the number of total cardiovascular events, but not adverse events leading to hospitalisation, disability or death(Reference Grenet, Le, Bejan-Angoulvant, Erpeldinger, Boussageon and Kassaï28). Among the elderly, intensive antihypertensive therapy reduced the risk of CVD mortality, but not the risk of stroke(Reference Takami, Yamamoto, Arima and Sakima29). Ultimately, intensive reduction in systolic blood pressure was only beneficial for the prevention of stroke recurrence, but not first occurrence(Reference Kitagawa, Yamamoto and Arima30).
Diabetes and disturbances in the glucose and insulin metabolism
Diabetes has been associated with increased CVD risk(31). Indeed, CVD is the most prevalent cause of morbidity and mortality in patients with diabetes(Reference Leon32), thus suggesting an important link between diabetes and CVD. Vascular complications in diabetes might be related to genetic polymorphisms related to nitric oxide production(Reference Dong, Ping, Wang and Zhang33). As demonstrated in Figure 2, systematic reviews of observational studies have reported associations between different markers of disturbances in the glucose and insulin metabolism with CVD. Glycaemic variability(Reference Pu, Lai and Yang34) was associated with poorer prognosis in coronary artery disease; fasting glucose(Reference Liao, Saver and Yeh35) with higher risk of major cardiovascular adverse events(Reference Zhao, Guo and Shi36–Reference Laichutai and Defronzo38); and fasting insulin(Reference Xun, Wu, He and He39,Reference Ruige, Assendelft, Dekker, Kostense, Heine and Bouter40) with coronary artery disease, but there was no significant association between the homeostatic model assessment for insulin resistance (HOMA-IR)(Reference Gu, Yang, Ying and Jin41) and stroke. Ultimately, an increase in C-peptide levels was associated with a rise in CVD mortality(Reference Andrade, Callo and Horta42).

Fig. 2. Diabetes and disturbances in the glucose and insulin metabolism associated with CVD risk, incidence and mortality.
Different stages and types of diabetes were also associated with increased CVD risk in previous systematic reviews of observational studies, including type 1 diabetes, which was associated with increased subclinical atherosclerosis(Reference Wang, Xu and Lv43), and type 2 diabetes with increased risk of stroke, heart failure and occlusive vascular mortality(Reference Lau, Lew, Borschmann, Thijs and Ekinci44–Reference Peters, Huxley and Woodward48), with some studies reporting differences between the sexes. For women, having type 2 diabetes was associated with a higher risk for coronary heart disease, stroke, cardiac death and all-cause mortality compared with men with type 2 diabetes(Reference Wang, Nie and Yu49); Moreover, gestational diabetes was related to increased cardiovascular events postpartum(Reference Kramer, Campbell and Retnakaran50), and pre-diabetes was associated with higher risk of major cardiovascular adverse events(Reference Liao, Saver and Yeh35).
Most of the systematic reviews and meta-analyses on this topic were based on observational study designs, and therefore were prone to residual confounding that may affect the associations between diabetes and risk of cardiovascular disease. Furthermore, significant heterogeneity across studies attributable to variability in the measurement of outcomes and adjustment for covariates(Reference Pu, Lai and Yang34,Reference Liao, Saver and Yeh35,Reference Lau, Lew, Borschmann, Thijs and Ekinci44,Reference Mitsios, Ekinci, Mitsios, Churilov and Thijs45,Reference Peters, Huxley and Woodward48,Reference Li, Song, Li, Liu, Sun and Yang51–Reference Zhang, Shao, Zhu, Ze, Zhu and Bi53) , subgroup comparisons(Reference Dong, Guan, Xu, Zhu, Zhang and Cheng54), and duration of follow-up(Reference Pu, Lai and Yang34,Reference Pulipati, Ravi and Pulipati55) , misclassification of diabetic cases(Reference Zhao, Guo and Shi36,Reference Mitsios, Ekinci, Mitsios, Churilov and Thijs45,Reference Pan, Lu, Lian, Liao, Guo and Zhang56) , and inconsistencies in defining conventional CVD risk factors(Reference Li, Song, Li, Liu, Sun and Yang51,Reference Wang, Ba, Cai and Xing52) were observed. Publication bias was found in some of the systematic reviews(Reference Pu, Lai and Yang34,Reference Pulipati, Ravi and Pulipati55,Reference Pan, Lu, Lian, Liao, Guo and Zhang56) , and the limited availability of data and/or studies prevented further use of meta-regression analysis(Reference Mitsios, Ekinci, Mitsios, Churilov and Thijs45,Reference Li, Song, Li, Liu, Sun and Yang51) .
Despite the limitations of observational studies, systematic reviews of experimental studies have supported a causal role of pharmacologically mediated glucose control in reducing the risk of non-fatal stroke, non-fatal myocardial infarction, hospitalisation for heart failure, and major adverse cardiac events(Reference Pulipati, Ravi and Pulipati55,Reference Malik, Yandrapalli, Goldberg, Jain, Frishman and Aronow57–Reference Kristensen, Rørth and Jhund61) , yet intensive glucose reductions did not appear to add extra benefits among type 2 diabetic patients with high risk of CVD(Reference Zhang, Liu, Zhang, Li and Tong62,Reference Barer, Cohen and Cukierman-Yaffe63) .
Dyslipidaemia
Traditional markers of lipid profile, including total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and triacylglycerols (TGL), are well-established risk factors for CVD and are often included in CVD prevention and management guidelines(Reference Piepoli, Hoes and Agewall64,Reference Arnett, Blumenthal and Albert65) . Recent reviews of observational studies have highlighted distinct relationships between these traditional markers and different cardiovascular diseases sub-types. For example, they have reported weak inverse associations between LDL-C levels and haemorrhagic stroke(Reference Ma, Na, Neumann and Gao66), but have not found significant associations between total cholesterol, LDL-C and HDL-C with ischaemic stroke(Reference Cui and Naikoo67) or death(Reference Deng, Li and Zhang68). In terms of TGL, reviews have shown detrimental associations between TGL and coronary heart disease(Reference Ye, Kong, Zafar and Chen69) and death(Reference Deng, Li and Zhang68), but mixed associations between TGL and ischaemic stroke in Western populations(Reference Cui and Naikoo67–Reference Ye, Kong, Zafar and Chen69). In patients with diabetes, the association between TGL and coronary heart disease became insignificant after adjustment for other lipid parameters(Reference Ye, Kong, Zafar and Chen69), which was not observed in studies with general population.
As shown in Figure 3, it has been suggested that additional lipid-related factors such as inherited lipid abnormalities (e.g. familial hypercholesterolemia), levels of apolipoprotein a-1, apolipoprotein b, lipoprotein (a) or apolipoprotein E4 isoform should also be considered in CVD risk assessment(70–Reference Sniderman, Williams and Contois75). Familial hypercholesterolaemia is a genetic condition that results in elevated LDL-C and potentially increased CVD risk(Reference Akioyamen, Genest and Shan76). Recent systematic reviews of observational studies have suggested associations between familial hypercholesterolaemia and atherosclerotic cardiovascular disease and non-vascular complications, including increased abdominal aortic aneurysm(Reference Akioyamen, Tu and Genest77), stroke and peripheral artery disease, with some associations losing significance when analyses were restricted to genetically confirmed cases (e.g. stroke and peripheral artery disease)(Reference Akioyamen, Tu and Genest77,Reference Anagnostis, Vaitsi, Mintziori, Goulis and Mikhailidis78) . The certainty of associations was limited by the paucity of studies available worldwide, in particular prospective studies(Reference Akioyamen, Tu and Genest77). In addition, the use of data at study level made it difficult to determine which risk factors were adjusted for in the pooled analysis(Reference Barer, Cohen and Cukierman-Yaffe63). In terms of lipoprotein a, a case control followed by meta-analysis supported an association between lipoprotein a and the risk of coronary disease(Reference Perrot, Moschetta and Boekholdt79). More recently, a review of observational studies reported detrimental associations between lipoprotein a and acute coronary syndrome, acute myocardial infarction and ischaemic stroke, but mixed findings related to CVD recurrence and death(Reference Kouvari and Panagiotakos80).

Fig. 3. Dyslipidaemia and lipid disorders associated with CVD risk, incidence and mortality.
In addition to inherited disorders of lipid metabolism, other advancements in this area include the exploration of the association between non-HDL-cholesterol, which has been associated with highest risk of ischaemic stroke (but not haemorrhagic) in men(Reference Wang, Ye and Pan81), increased CVD risk in the general population and individuals with type 2 diabetes, but not with CVD mortality(Reference Cao, Yan and Guo82). Non-HDL was also considered a better predictor of coronary heart disease than LDL-C on its own(Reference Michael, Parisis and Best83). Moreover, the additional value of the heterogenous population of LDL and HDL particles (LDL-P and HDL-P, respectively) has also been explored. For instance, a systematic review reported associations between LDL-P or small LDL particles and CVD risk(Reference Ip, Lichtenstein, Chung, Lau and Balk84). In addition, in a meta-analysis of two prospective studies, HDL-C was not associated with atherosclerotic cardiovascular disease, myocardial infarction or stroke, but HDL-P was inversely associated with atherosclerotic cardiovascular disease(Reference Singh, Ayers and Rohatgi85), which was also identified in a previous systematic review of cohorts and case–control studies(Reference Wu, Fan, Tian, Liu and Liu86). An additional meta-analysis of prospective studies has also shown differences in associations between HDL and coronary heart disease (CHD) risk based on ApoC-III binding with HDL(Reference Jensen, Aroner and Mukamal87). A systematic review of observational studies has described that higher monocyte-to-HDL ratio on admission in patients with ST-elevation myocardial infarction who underwent primary percutaneous coronary intervention was significantly associated with higher major adverse cardiovascular events and in-hospital mortality(Reference Villanueva, Tiongson, Ramos and Llanes88). A systematic review of observational and experimental studies has also reported negative associations between cholesterol efflux and risk of coronary artery disease or acute coronary syndrome, but not between stroke or myocardial infarction(Reference Ye, Xu, Ren and Peng89). Reverse cholesterol transport refers to the mechanism by which accumulated cholesterol is moved from the vessel wall to the liver for excretion. An important step in this pathway is cholesterol efflux, by which macrophages within the vessel wall secrete cholesterol outside cells. Moreover, a Mendelian randomisation study supported a causal association between remnant cholesterol levels and ischaemic heart disease(Reference Varbo, Benn, Tybjærg-Hansen, Jørgensen, Frikke-Schmidt and Nordestgaard90), yet only Caucasian subjects were included. Overall, issues related to limited number/quality of studies(Reference Cui and Naikoo67,Reference Deng, Li and Zhang68) , measurement (e.g. single versus repeated measures and fasting versus post-prandial)(Reference Ye, Kong, Zafar and Chen69,Reference Wang, Ye and Pan81,Reference Cao, Yan and Guo82,Reference Jensen, Aroner and Mukamal87) or cut-offs used(Reference Deng, Li and Zhang68,Reference Wang, Ye and Pan81,Reference Cao, Yan and Guo82,Reference Villanueva, Tiongson, Ramos and Llanes88) and adjustment for confounders(Reference Cui and Naikoo67,Reference Ye, Kong, Zafar and Chen69,Reference Villanueva, Tiongson, Ramos and Llanes88) were raised as they could impact the strength of the associations between lipid markers and CVD.
In terms of experimental studies, previous systematic reviews have shown that lipid-lowering (statins and non-statins) interventions reduce clotting tendency(Reference Schol-Gelok, Morelli and Arends91), stroke(Reference Banach, Shekoohi, Mikhailidis, Lip, Hernandez and Mazidi92) and incidence of major cardiovascular events in high-risk patients(Reference Dicembrini, Giannini, Ragghianti, Mannucci and Monami93), with the reduction of coronary atherosclerosis being associated with reductions in LDL-C and non-HDL-C(Reference Masson, Lobo and Siniawski94). Interventions aiming to reduce LDL-C to very low levels showed additional benefits compared with standard targets(Reference Navarese, Robinson and Kowalewski95,Reference Shin, Chung and Jang96) and reduced cardiovascular mortality in patients with higher baseline LDL-C levels(Reference Masson, Lobo and Siniawski94), though at the cost of increasing the risk of intracerebral haemorrhage(Reference Cheng, Qiao and Jiang97), which is perceived to be outweighed by the benefits it promotes in other stroke sub-types(Reference Arnett, Blumenthal and Albert65). Decisions on the intensity of LDL-C targets should be tailored to individuals’ CVD risk.
Obesity
Figure 4 shows the association between obesity and CVD risk, events and mortality. Different markers of obesity have been associated with increased acute myocardial infarction(Reference Li, Yao and Yang98), sudden cardiac death(Reference Chen, Deng and Li99,Reference Aune, Schlesinger, Norat and Riboli100) , stroke(Reference Price, Wright and Green101,Reference Liu, Zhang and Liu102) , deleterious vascular outcomes(Reference Hong, Lin and Gu103) and cardiovascular mortality(Reference Jayedi and Shab-Bidar104). For instance, specific adipocytokines, which are biologically active mediators secreted by the adipose tissue, such as adiponectin(Reference Scarale, Fontana, Trischitta, Copetti and Menzaghi105) and visfatin(Reference Yu, Li, Huang and Cai106) have been associated with higher coronary artery disease and CVD mortality. In addition, a prospective cohort study of people with obesity and a normal metabolic profile, sometimes referred to as having ‘metabolically healthy obesity’ showed a substantially higher risk of developing diabetes and atherosclerotic CVD and increased all-cause mortality compared with people with metabolically healthy non-obesity(Reference Zhou, Macpherson and Gray107). The increased CVD risk among metabolically healthy overweight and obese could be explained by the high risk of future metabolic dysregulation or obesity-related health consequences(Reference Yeh, Chen, Tsai, Lin, Liu and Chien108,Reference Barzin, Valizadeh, Serahati, Mahdavi, Azizi and Hosseinpanah109) .

Fig. 4. Obesity associated with CVD risk, incidence and mortality.
Systematic reviews of prospective observational studies have also shown that weight gain was associated with myocardial infarction, coronary heart disease, stroke and CVD incidence(Reference Jayedi, Rashidy-Pour, Soltani, Zargar, Emadi and Shab-Bidar110). On the other hand, there is a weight loss paradox in CVD mortality(Reference Chen, Yang, Wang, Li, Ying and Yuan111–Reference Zomer, Gurusamy and Leach114), which seems to be driven by unintentional weight loss(Reference De Stefani, Pietraroia, Fernandes-Silva, Faria-Neto and Baena115). Limitations of these performed systematic reviews include the fact that the majority of studies used BMI as the only indicator of adiposity/obesity(Reference Hsueh, Yeh and Lin116–Reference Di Angelantonio, Bhupathiraju and Wormser123). BMI does not distinguish between weight associated with muscle and weight associated with fat. Furthermore, it does not take into account the difference in fat storage across diverse ethnicities. In some studies, there was no direct measure of body composition(Reference Liu, Zhang and Liu121,Reference Yusuf, Hawken and ⓞunpuu124,Reference Chen, Yang, Wang, Li, Ying and Yuan125) , thus raising concerns regarding the use of convenient indicators of obesity to accurately predict CVD risk. Another important limitation was the lack of adjustment for effect modifiers and confounding factors, such as age, sex, physical activity, smoking status, socio-economic status and medical treatment(Reference Aune, Schlesinger, Norat and Riboli100,Reference Barzin, Valizadeh, Serahati, Mahdavi, Azizi and Hosseinpanah109,Reference Karahalios, English and Simpson112,Reference Zomer, Gurusamy and Leach114,Reference De Stefani, Pietraroia, Fernandes-Silva, Faria-Neto and Baena115,Reference Jayedi and Shab-Bidar120,Reference Liu, Zhang and Liu121,Reference Yusuf, Hawken and ⓞunpuu124,Reference Kane, Mehmood and Munir126–Reference Yu, Li, Huang and Cai129) . These aspects can influence the meta-analyses’ results and must be considered when interpreting the results. In addition, studies that evaluated the influence of genetic aspects did not consider ethnicity or genetic ancestry, thus having implications for generalising findings(Reference Lv, Zhang and Fan130,Reference Chen, Li and Yi131) . Ultimately, sources of bias, including publication bias, small-study effect and evidence of high heterogeneity between studies were also identified. Sources of heterogeneity were often associated with differences in study characteristics, definitions and measurement of outcomes and sample size, and background characteristics of populations from which the studies were derived(Reference Yeh, Chen, Tsai, Lin, Liu and Chien108,Reference Hsueh, Yeh and Lin116,Reference Jayedi and Shab-Bidar120,132–Reference Cao, Yu and Xiong134) .
We also see supportive evidence from Mendelian randomisation studies suggesting a causal link between obesity, in particular central obesity, with increased peripheral arterial disease(Reference Huang, Xu and Xie135), coronary arterial disease(Reference Lv, Zhang and Fan130) and CVD(Reference Riaz, Khan and Siddiqi136,Reference Kelley, Kelley and Stauffer137) . In addition to a possible causal link of central obesity in CVD risk, measures of central obesity, such as waist circumference and waist-to-hip ratio, have also been considered better predictors of CVD risk than BMI(Reference Darbandi138). Although not completely elucidated, different mechanisms have been cited to explain the important role that central obesity plays in CVD, such as promoting insulin resistance, inflammation and oxidative stress, which contribute to an atherogenic state(Reference Ross, Neeland and Yamashita139–Reference Gruzdeva, Borodkina, Uchasova, Dyleva and Barbarash141).
Systematic reviews of RCTs have also investigated the impact of weight-reducing diets combined or not with physical activity and pharmacological approaches to weight loss. Pharmacological interventions resulted in a significant decrease in CVD mortality(Reference Kane, Mehmood and Munir126). One review explored the impact of weight loss interventions (often low-fat diets) and found no significant impact on CVD incidence and mortality, yet this study was not able to establish whether other forms of diet would provide different results(Reference Ma, Avenell, Bolland and Robertson142), but this will be discussed further in the diet section of this paper. In terms of bariatric surgery, a systematic review found only one RCT that showed no effect of surgery on heart failure incidence(Reference Berger, Meyre and Blum143). Remaining evidence in this area comes from observational studies(Reference Kuno, Tanimoto, Morita and Shimada144–Reference Zhou, Yu and Li146) that show associations among surgery and reduced incidence of heart failure and mortality(Reference Singh, Subramanian and Adderley147) and coronary artery disease deaths(Reference Kanji, Wong, Akioyamen, Melamed and Taylor148), for example. In a number of RCTs, bariatric surgery has also resulted in long-term weight reduction, more often driven by reduced food intake, yet additional mechanisms such as gastric emptying and changes in appetite hormones are also involved. RCTs have also reported improvements in other cardiovascular risk factors, such as hypertension, dyslipidaemia and type 2 diabetes(Reference Beamish, Olbers, Kelly and Inge149).
Emerging risk factors for CVD
It has been suggested that conventional risk factors for CVD fail to explain at least 25 % of new CVD cases, which prompted the interest in exploring the role of emerging risk factors(Reference Vilahur, Badimon, Bugiardini and Badimon150). Despite criticism(Reference Beaglehole and Magnus151), studying emerging risk factors for CVD has the potential to contribute to our understanding of the pathophysiological mechanisms of CVD and identify new areas for prevention and targets for treatment(Reference Lacey, Herrington, Preiss, Lewington and Armitage152).
Inflammation
Figure 5 illustrates the increasing interest in the association between inflammation and CVD risk and mortality. Systematic reviews of observational studies have shown that systemic inflammatory conditions such as rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis and ankylosing spondylitis are associated with increased subclinical atherosclerosis and risk of developing cardiovascular disease(Reference Yong, Sanguankeo and Upala153–Reference Mathieu, Couderc, Tournadre and Soubrier156). In addition, endometriosis, considered an inflammatory disease, has also been associated with increased risk of lifelong cardiovascular events, including myocardial infarction, independent of demographic and lifestyle factors(Reference Tan, Taskin and Iews157). Localised inflammation has also been linked to vascular pathology. For instance, a meta-analysis of observational studies revealed a significant relationship between periodontitis and peripheral arterial disease(Reference Yang, Zhao, Cai, Shi, Wen and Xu158). Ultimately, inflammation triggered by pathogens has also been linked to increased risk of cardiovascular and cerebrovascular events, specifically infections with Helicobacter pylori, Chlamydia pneumonia and Mycoplasma pneumonia (Reference Khademi, Vaez, Momtazi-Borojeni, Majnooni, Banach and Sahebkar159).

Fig. 5. Inflammation associated with CVD risk, incidence and mortality.
Different markers of inflammation have been associated with increased CVD risk, although not all the identified relationships were proven to be causal. For instance, a reliable marker of systemic inflammation, C-reactive protein (CRP), and high-sensitivity C-reactive protein (hs-CRP) were associated with increased risk of major cardiovascular events among patients with peripheral artery disease in systematic reviews and meta-analysis of observational studies(Reference Singh, Morris, Smith, Moxon and Golledge160,Reference Kaptoge, Di Angelantonio and Lowe161) , with increased risk of in-hospital mortality in patients with aortic dissection(Reference Hsieh, Henry, Hsieh, Maruna, Omara and Lindner162) and with cardiovascular and all-cause mortality in type 2 diabetes patients; this result was independent of study design, follow-up duration of the patients and the presence of pre-existing cardiovascular risk factors(Reference Tian, Tian and Wang163). Despite supportive evidence from observational studies, a Mendelian randomisation study has reported that variation in hs-CRP was not associated with CVD(Reference Dehghan, Dupuis and Barbalic164), suggesting that hs-CRP might be a risk marker but not a risk factor. Additional markers further explored include: monocyte chemoattractant protein-1 or intercellular adhesion molecule 1, which is a marker of vascular inflammation that has been correlated with ischaemic stroke, coronary artery disease and myocardial infarction(Reference Georgakis, Malik and Björkbacka165,Reference Su166) ; erythrocyte distribution width (RDW) is a novel prognostic marker that may reflect an underlying inflammatory state and has been shown to predict adverse outcomes in CVD patients(Reference Song, Hua and Dornbors167); and neutrophil-to-lymphocyte ratio (NLR), which identifies subclinical inflammation and was identified as a predictor of hospitalisation and long-term prognosis in patients with ST-elevation myocardial infarction after percutaneous coronary intervention(Reference Zhang, Diao and Qi168).
Inflammation-associated immune response also seems to play a critical role in CVD progression. Pro-inflammatory cytokines such as T-helper cell 1 (Th1) and T-helper cell 2 were positively linked with retinopathy and cardiovascular complications(Reference Mahlangu, Dludla and Nyambuya169). One of the most important pro-inflammatory cytokines, tumour necrosis factor-alpha (TNF-α), was shown to contribute to extensive arterial disease(Reference Knowles, Nadeem and Chowienczyk170). Moreover, up-regulation of the TNF receptors 1 and 2 was positively associated with CVD events and mortality in patients with type 2 diabetes(Reference Cheng, Fei, Saulnier and Wang171).
Myeloperoxidase, a leucocyte-derived enzyme that quantifies inflammatory activity from the luminal aspect of the arterial wall, was shown to be positively associated with increased risk of mortality in patients with systemic CVD, but not associated with myocardial infarction or major adverse cardiac events(Reference Kolodziej, Abo-Aly, Elsawalhy, Campbell, Ziada and Abdel-Latif172). Serum amyloid A, an acute-phase protein involved in the pathogenesis of atherosclerosis, was associated with increased risk of coronary heart disease, especially for patients older than 55 years(Reference Zhou, Lu, Wang and Chen173). Systematic reviews of observational studies have shown that interleukin 6 (IL-6) is present at higher circulating levels in CVD patients than in non-CVD controls(Reference Zhang, Li, Zhao, Pan and Zhang174). In addition, a Mendelian randomisation study indicated that genetic variants which lead to higher circulating concentrations of IL-6 receptors (IL-6R) (and consequently less IL-6 cell signalling and lower circulating CRP) appear to be protective against CHD(Reference Sarwar, Butterworth and Freitag175).
RCTs have shown mixed results on the impact of anti-inflammatory therapies (e.g., non-TNF targeting biologics and small molecules approved for the treatment of inflammatory arthritis such as tocilizumab, abatacept and anakinra) on atherosclerosis, stroke, myocardial infarction and cardiovascular disease(Reference Ursini, Ruscitti, Caio, Manfredini, Giacomelli and De Giorgio176). Although it has been hypothesised that groups of patients who have a significant inflammatory component contributing to their cardiovascular disease might benefit from anti-inflammatory strategies(Reference Jones and Patel177), such evidence comes mostly from observational studies. It has been suggested that different immunomodulatory treatments might be needed for different atherosclerotic stages(Reference Welsh, Grassia, Botha, Sattar and Maffia178), but more studies are necessary to determine specific targets that contribute to increased CVD risk in patients with local and systemic inflammation.
Oxidative stress
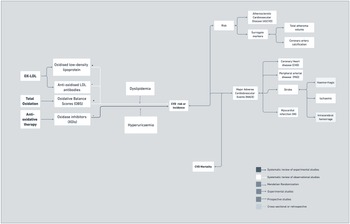
Most of the evidence linking oxidative stress to CVD comes from observational studies. In systematic reviews, oxidised LDL (ox-LDL) levels were associated with carotid plaques, carotid media thickness and atherosclerotic cardiovascular disease, yet associations were not consistent across studies(Reference Gao and Liu179). In addition, a review of prospective studies showed that immunoglobulin M anti-ox-LDL antibodies offer some protection from more severe coronary artery disease and possibly cardiovascular events(Reference van den Berg, Vroegindewey and Kardys180). Emerging evidence from one systematic review further linked oxidative balance scores, which evaluate the global balance of individuals’ oxidation–reduction status, with CVD risk(Reference Hernández-Ruiz, García-Villanova, Guerra-Hernández, Amiano, Ruiz-Canela and Molina-Montes181). Additionally, a critical review suggested that NADPH oxidases might play an important role in cardiovascular disease occurrence and development(Reference Zhang182). In terms of experimental studies, a recent systematic review of randomised studies showed that addition of oxidase inhibitors to conventional angiotensin-converting enzyme inhibitors may potentially result in improved survival free from major adverse cardiovascular events in post-acute myocardial infarction patients (Figure 6)(Reference Borghi, Omboni and Reggiardo183).

Fig. 6. Oxidative stress associated with CVD risk, incidence and mortality.
Haemostatic and fibrinolytic disturbances
The haemostatic system prevents bleeding and haemorrhage following any form of injury to the endothelium. In the context of CVD, the rupture of the atheromatous plaque triggers coagulation processes within the arterial lumen, which leads to thrombus formation and blood clot obstructing blood flow, thereby causing tissue death(Reference Stehouwer, Pieters, de Maa, Stanner and CoeSara184).
As shown in Figure 7, several markers of coagulation processes have been studied in previous years. For instance, a recent meta-analysis of prospective studies followed by a Mendelian randomisation study identified thirteen genetic loci with modest contributions to plasma levels of factor VIII (FVIII) or von Willebrand factor (vWF)(Reference Sabater-Lleal, Huffman and de Vries185), which have also shown to be associated with the risk of venous thromboembolism(Reference Sabater-Lleal, Huffman and de Vries185), coronary artery and heart disease(Reference Fan, Zhu and Zhao186,Reference Willeit, Thompson, Aspelund and Stoll187) and major adverse cardiovascular events(Reference Fan, Zhu and Zhao186). For fibrinogen, a systematic review of prospective studies reported that fibrinogen levels improved CVD risk prediction in men but not in women(188). However, a more recent meta-analysis of prospective studies showed that there was no evidence for a causal role of fibrinogen in coronary artery disease, stroke and venous thromboembolism(Reference Sabater-Lleal, Huang and Chasman189). Tissue plasminogen activator (t-PA) is a thrombolytic protease produced mainly by vascular endothelial cells, that converts inactive plasminogen into active plasmin, which then degrades fibrin complexes, a major component of a thrombus. A review of prospective studies showed that t-PA antigen was log-linearly related to coronary heart disease risk(Reference Willeit, Thompson, Aspelund and Stoll187). In addition to t-PA, D-dimer and vWF, which are markers of coagulation, were more weakly associated with incident coronary heart disease than markers of inflammation (IL-6 and CRP) and lipid metabolism (TGL, lipoprotein (a) and total cholesterol) in a systematic review of prospective studies(Reference Willeit, Thompson, Aspelund and Stoll187). Observational associations between coagulation markers and CVD risk were limited by a number of factors, including small number of studies available, incomplete information(Reference Fan, Zhu and Zhao186,188) and measurement issues (e.g. diurnal or seasonal fluctuations)(Reference Fan, Zhu and Zhao186–188).

Fig. 7. Haemostatic and fibrinolytic disturbances associated with CVD risk, incidence and mortality.
In contrast to t-PA, plasminogen activator inhibitor-1 (PAI-1) is a member of the serine protease inhibitor family which inhibits fibrinolysis. The thrombosis process is determined by the balance between t-PA and PAI-1, with increased PAI-1 levels in plasma inducing a hypercoagulable state(Reference Lucore and Sobel190). Systematic reviews have shown that PAI-1 levels were an independent predictor of a first CVD event among individuals with no prior CVD(Reference Song, Burgess and Eicher191). Furthermore, elevated PAI-1 antigen levels were associated with major adverse cardiac events (myocardial infarction and cerebrovascular events, including stroke and transient ischaemic attacks) and death in populations with and without established CVD. In addition, elevated PAI-1 antigen levels were associated with all-cause mortality(Reference Jung, Motazedian and Ramirez192). Another study, using observational data and Mendelian randomisation, has shown the causal effect of PAI-1 on CHD onset, potentially mediated by blood glucose dysfunction, suggesting that the fibrinolysis pathway may be a good target for CHD treatment(Reference Song, Burgess and Eicher191).
In terms of experimental studies, recent systematic reviews of RCTs have shown that different anti-coagulant therapies (factor Xa inhibitors such as apixaban, edoxaban and rivaroxaban) reduced the occurrence of ischaemic stroke(Reference Bruins Slot and Berge193,Reference Es, cobar, Martí-Almor, Pérez Cabeza and Martínez-Zapata194) and its severity(Reference Meinel, Frey and Arnold195), of embolism(Reference Mai, Guay and Perreault196–Reference Gupta, Um and Pandey198) and of major adverse cardiovascular events(Reference Liu, Lu and Chen199). Factors such as individuals’ age and BMI could modify the treatment effect of anti-coagulation but were not explored in all studies for anti-coagulation therapies.
The link between risk factors and endothelial dysfunction, an intermediary marker of CVD
The vascular endothelium plays a vital role in the pathogenesis of cardiovascular disease. We found evidence linking conventional CVD risk factors, such as hypertensive disorders(Reference Kirollos, Skilton, Patel and Arnott23), obesity and diabetes(Reference Loader, Khouri and Taylor200) to systemic endothelial dysfunction, leading to CVD. Chronic diseases such as non-alcoholic fatty liver disease have also been associated with an increased risk of atherosclerotic cardiovascular disease, in a mechanism involving endothelial dysfunction(Reference Zhou, Zhou and Wu201). Albuminuria, which is an early marker of kidney disease, has also been associated with cerebral small vessel disease, which makes it particularly important as a biomarker in the evaluation of brain microvascular damage(Reference Georgakis, Chatzopoulou, Tsivgoulis and Petridou202). The release of endothelial cell-derived substances such as nitric oxide, a potent vasodilator, contributes to vascular health. A non-invasive method of assessing endothelial function in peripheral vessels is brachial artery flow-mediated dilatation (FMD)(Reference Thijssen, Bruno and van Mil203).
During the test, a rapid decrease in blood flow is induced by inflating an arm cuff to supra-systolic pressure for 5 min. This ischaemic period results in increased production of nitric oxide and other vasoactive molecules from the endothelial cells. Upon pressure release, the increase in flow known as hyperaemia, which induces shear stress, results in vasodilatation mediated by nitric oxide diffusing into the vascular smooth muscle cells. These changes can be monitored in real time using a Doppler ultrasound. Therefore, brachial artery FMD is considered a measure of endothelium- and nitric oxide-dependent vasodilation(Reference Tousoulis, Antoniades and Stefanadis204). Several meta-analyses have suggested that impairment of brachial FMD is significantly associated with future cardiovascular events(Reference Ras, Streppel, Draijer and Zock205–Reference Inaba, Chen and Bergmann207). The strength of association of FMD and future CVD events was found to be higher in patients with established CVD, suggesting that FMD may be more useful in screening for recurrent CVD events in patients at high risk(Reference Xu, Arora and Hiebert208).
Recent advances in this area have also highlighted a shared mechanistic pathway involving inflammation and endothelium oxidation in endothelial dysfunction(Reference Griessenauer, Farrell and Sarkar209,Reference Xu, Zhan and Mai210) . For instance, Single Nucleotide Polymorphisms (SNPs) involved in processes related to endothelial and vascular health such as vascular endothelial growth factor receptor, platelet/endothelial cell adhesion molecule, endothelial nitric oxide synthase and endothelin-1 contribute to local and systemic vascular disorders(Reference Wang, Ge, Peng and Wang211–Reference Ali215), yet studies had small sample sizes, were limited in number or did not account for gene–environment interaction.
The carotid intima–media thickness (CIMT) is used to diagnose the extent of carotid atherosclerotic vascular disease by using ultrasound which measures the thickness of the inner two layers of the carotid artery: the intima and media. CIMT changes precede plaque formation in carotid atherosclerosis, so this non-invasive measurement can accurately describe the process of arterial wall changes due to atherosclerosis and can be used to monitor apparently healthy and at-risk populations(Reference de Groot, Hovingh and Wiegman216). Systematic review and meta-analysis have also established that increased CIMT is a reliable marker of the progression of coronary artery disease, of increased risk of stroke in older adults without a history of cardiovascular disease and of cerebral infarction and atherosclerosis throughout the body(Reference Katakami, Kaneto and Shimomura217). Furthermore, increased CIMT was shown to be an independent predictor of myocardial infarction and CVD(Reference Sabeti, Schlager and Exner218). Several other longitudinal studies showed that CIMT measurements are a validated surrogate endpoint for atherosclerosis and vascular disease risk, suggesting that CIMT can provide additional information that cannot be obtained based on the assessment of conventional cardiovascular risk factors alone (Figure 8)(Reference Lorenz, Markus, Bots, Rosvall and Sitzer219).

Fig. 8. Endothelial dysfunction associated with CVD risk, incidence and mortality.
The interconnections between CVD risk factors
Intergenerational perspective
The hypothesis that fetal, infant and childhood conditions affect CVD risk later in life is not new(Reference Barker and Fall220). In recent years, systematic reviews of observational studies provided an indication that exposure to famine (particularly when coupled with exposure to nutritional excess after birth), higher maternal weight(Reference Pullar, Wickramasinghe and Demaio221), maternal diabetes(Reference Pathirana, Lassi, Roberts and Andraweera222), maternal dyslipidaemia(Reference Wang, Moore and Subramanian223), maternal hypertension(Reference Andraweera and Lassi224) and maternal polycystic ovarian syndrome(Reference Gunning, Sir Petermann and Crisosto225) were associated with offspring’s risk of congenital heart defects and CVD events. There is also indication that low birth weight and prematurity are associated with greater CVD and CV event risk(Reference Markopoulou, Papanikolaou, Analytis, Zoumakis and Siahanidou226,Reference Knop, Geng and Gorny227) . Some researchers also hypothesise that adverse intra-uterine conditions result in both structural changes in the organs along with increased risk of metabolic disturbances, such as obesity, hypertension, diabetes and dyslipidaemias, all of which impact CVD risk(Reference Menendez-Castro, Rascher and Hartner228). As presented in Figure 9, the majority of the evidence on the role of fetal and childhood conditions comes from observational study designs, meaning that residual or unmeasured factors may confound the association between fetal and childhood conditions with future CVD risk. In addition, study heterogeneity, which in some cases prevented meta-regression analysis(Reference Kolding, Eken and Uldbjerg229), as well as recall bias(Reference Mintjens, Menting, Daams, van Poppel, Roseboom and Gemke230), selection bias, citation bias(Reference Knop, Geng and Gorny231) and short follow-up, which prevented further stratified analysis or subgroup comparisons such as sex- or age-specific relationships(Reference Pullar, Wickramasinghe and Demaio221,Reference Pathirana, Lassi, Roberts and Andraweera232) in future CVD risk, were important limitations. A small number of intervention studies have supported that macro- and micronutrient supplementation in mothers during the antenatal period resulted in lower incidence of type 2 diabetes and hypertension in childhood, but longer follow-up is still needed(Reference Stewart, Christian, Schulze, LeClerq, West and Khatry233,Reference Devakumar, Chaube and Wells234) .

Fig. 9. Intergenerational perspective associated with CVD risk, incidence and mortality.
Integration of multiple biological pathways
We find many examples that illustrate how multiple physiological pathways are integrated in CVD risk. For instance, one previous Mendelian randomisation study with 90 000 Danish individuals suggested that the relationship between obesity and ischaemic heart diseases was partly mediated through non-fasting remnant cholesterol (7 %), which is the cholesterol content of triacylglycerol-rich lipoproteins, low-density lipoprotein cholesterol (8 %) and elevated blood pressure (7 %)(Reference Varbo, Benn, Smith, Timpson, Tybjærg-Hansen and Nordestgaard235). A more recent two-sample Mendelian randomisation study with a larger number of participants also supported the involvement of triacylglycerol levels and further identified an important role of poor glycaemic control in the risk of coronary heart diseases(Reference Xu, Borges, Hemani and Lawlor236). Body composition changes typical of obesity (e.g. visceral fat and ectopic fat), as well as pro-inflammatory cytokines produced by the adipose tissue, might influence CVD risk via mechanisms related to changes in lipid profile (e.g. LDL-C, HDL-C, triacylglycerols and remnant cholesterol), insulin resistance and hypertension, but also through pro-inflammatory (e.g. increase in TNF-α and IL-6), pro-thrombotic (e.g. increase in PAI-1) and pro-oxidative states (e.g. ox-LDL, reduced nicotinamide adenine dinucleotide and reactive oxygen species)(Reference Varbo, Benn, Smith, Timpson, Tybjærg-Hansen and Nordestgaard235–Reference Cercato and Fonseca238), as well as through haemodynamic and structural changes of the heart, and cardiac functions(Reference Carbone, Canada, Billingsley, Siddiqui, Elagizi and Lavie239).
Obesity is not the only component interacting with other risk factors for CVD risk. For instance, a recent umbrella review has supported a causal role of higher birth weight, BMI, waist circumference and systolic blood pressure in diabetes risk(Reference Bellou, Belbasis, Tzoulaki, Evangelou and Nerurkar240), which have been discussed in previous sections of this paper as being relevant risk factor for CVD. In addition, a Mendelian randomisation study has supported the existence of shared genes between type 2 diabetes and coronary heart disease, yet mediation analyses are still needed to disentangle the effects of type 2 diabetes from those related to obesity in the context of CVD risk(Reference Goodarzi and Rotter241).
The role of natural and built human environments
Beyond interactions between genes and physiological systems, characteristics of the human environment directly or indirectly affect CVD risk, such as circadian rhythms, seasons of the year, sunlight exposure, altitude, noise, pollution, green spaces, socio-economic deprivation, social networks, nutrition and physical activity(Reference Bhatnagar242). For example, exposure to air pollution might contribute to increased CVD risk via increased oxidative stress, followed by increased inflammatory and coagulation responses, which impact atherosclerosis, vascular and endothelial functions(Reference Al-Kindi, Brook, Biswal and Rajagopalan243). Exposure to air pollution might be particularly worrisome for susceptible individuals, such as those with underlying atherosclerosis (or predisposing conditions), who are at higher risk of cardiovascular event or deaths in response to pollution exposure, when compared with those who are otherwise healthy(Reference Al-Kindi, Brook, Biswal and Rajagopalan243). Thus, understanding how different characteristics of human environments, including diet, interact with physiological systems and affect CVD risk is necessary to improve CVD prediction and management.
The role of diet on CVD risk
Nutrient-based approach
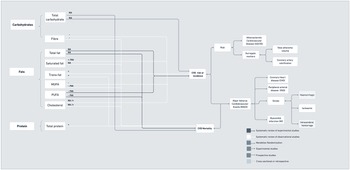
Diet is one component that moderates or amplifies CVD risk. As presented in Figure 10a, more robust evidence around macronutrients and CVD risk is available for the role of dietary fats, potentially due to the established relationship between circulating cholesterol levels and cholesterol-lowering interventions on CVD risk(Reference Tzoulaki, Elliott, Kontis and Ezzati244). However, evidence on the role of dietary cholesterol has been mixed. For instance, one systematic review and meta-analysis of cohort studies found that cholesterol intake was not associated with coronary artery disease, ischaemic stroke or haemorrhagic stroke(Reference Berger, Raman, Vishwanathan, Jacques and Johnson245). On the contrary, a more recent meta-analysis of prospective studies in the United States found that a higher intake of dietary cholesterol was associated with a higher risk of CVD incidence and mortality(Reference Zhong, Van Horn and Cornelis246). In addition, evidence to date is not supportive of a link between total fat intake and risk of coronary heart disease or CVD mortality. For specific lipid components, such as saturated fat, current observational studies do not show a relationship with coronary heart disease or CVD mortality either; the only exception seems to be a relationship with the incidence of ischaemic stroke. More consistent findings exist on the association between trans fatty acids and coronary heart disease and CVD mortality, thus justifying calls for trans-fat ban(247). In experimental studies, total intake of polyunsaturated fatty acids is also not associated with coronary heart disease or CVD mortality, and conflicting evidence remains on individual associations between n-3 and n-6 polyunsaturated fatty acids.

Fig. 10. (a) Macronutrients associated with CVD risk, incidence and mortality.

Fig. 10. (b) Micronutrients associated with CVD risk, incidence and mortality.

Fig. 10. (c) Foods associated with CVD risk, incidence and mortality.

Fig. 10. (d) Dietary patterns associated with CVD risk, incidence and mortality.
In 1953, a high intake of n-3 polyunsaturated fatty acids (n-3 PUFA) in the Greenland Eskimo population was associated with a relatively low incidence of CHD, and since then there is growing interest in the use of n-3 PUFA as therapeutic agents in CVD(Reference Bang and Dyerberg248). Most well-known sources of n-3 PUFA are marine-based fish and fish oil that contain eicosapentaenoic acid and docosahexaenoic acid. In reviews of prospective studies, increased n-3 PUFA intake was associated with a lower risk of fatal myocardial infarction or major cardiovascular events(Reference Chen, Yang, Eggersdorfer, Zhang and Qin249,Reference de Roos, Mavrommatis and Brouwer250) , yet mixed findings emerge from RCTs(Reference Chareonrungrueangchai, Wongkawinwoot, Anothaisintawee and Reutrakul8). Despite this, there is strong evidence from RTCs that replacing saturated fatty acids (commonly found in animal-based foods like butter, cheese, fatty meat) with n-3 PUFA reduces the risk of CVD events and coronary mortality(Reference Hooper, Summerbell, Thompson and Hooper251). The link between n-3 and CVD can be explained by the cardioprotective actions attributed to n-3 PUFA in mechanisms involving anti-inflammatory, anti-atherogenic, anti-arrhythmic, anti-thrombotic and antioxidant functions(Reference Kones, Howell and Rumana252).
More recently, increased attention has been paid to the relevance of other macronutrients, such as carbohydrates and proteins, for CVD incidence(Reference Ho, Gray and Welsh253). Similar to the evidence on fats, total intake of carbohydrates was not associated with CVD incidence and mortality as shown in a meta-analysis of large prospective studies conducted in North America and Europe(Reference Noto, Goto, Tsujimoto and Noda254). Rather, evidence to date suggests that the quality of carbohydrates might be more important than quantity. There is supporting evidence on the role of dietary fibre, with prospective studies and RCTs reporting associations between fibre intake and improvements in a number of physiological CVD risk factors(Reference Reynolds, Mann, Cummings, Winter, Mete and Te Morenga255) and coronary heart disease and CVD incidence(Reference Threapleton, Greenwood and Evans256).
Figure 10b shows that a number of micronutrients have been tested in experimental studies for their potential role in CVD prevention. There are a number of studies that refute benefits of single nutrients, such as from vitamin D and C, for decrease in CVD risk, major adverse events and mortality(Reference Barbarawi, Kheiri and Zayed257). However, there is some supportive evidence of a potential benefit of folic acid and B vitamins in stroke and CVD prevention(Reference Jenkins, Spence and Giovannucci258). In addition, a recent Mendelian randomisation study showed that genetically predicted vitamin E levels were associated with weak protection in terms of coronary artery disease and myocardial infarction, which could potentially be related to shared genetic pathways between vitamin E and lipid metabolism(Reference Wang and Xu259).
Food-based approach
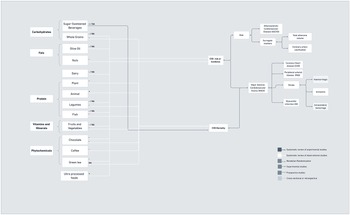
In recognition that individuals eat whole foods and not isolated nutrients, studies have also explored the role of specific food groups or individual foods in CVD risk, which are depicted in Figure 10c. For instance, a recent systematic review of observational and experimental studies showed that olive oil, which is high in monounsaturated fatty acids, was associated with decreased risk of stroke, but not coronary heart disease. Also, in observational studies, consumption of nuts, which are high in polyunsaturated fats, was associated with both reduced coronary heart disease and stroke(Reference Chareonrungrueangchai, Wongkawinwoot, Anothaisintawee and Reutrakul8). Also, foods that are high in simple carbohydrates, such as sugar-sweetened beverages, were associated with increased coronary heart disease incidence in previous meta-analysis of prospective studies(Reference Xi, Huang, Reilly, Li, Zheng and Barrio-Lopez260), and whole grains, which are high in complex carbohydrates, were associated with decreased CVD mortality and risk of coronary heart disease, but not stroke(Reference Chareonrungrueangchai, Wongkawinwoot, Anothaisintawee and Reutrakul8).
In terms of protein-rich foods, such as meat, fish, legumes and dairy, it might be difficult to disentangle the effect of any particular nutrient on CVD risk, incidence or mortality. For instance, although higher total protein intake has been associated with a higher risk of CVD mortality and non-stroke CVD mortality in a recent meta-analysis of prospective studies (Figure 10a), this association was mainly driven by animal protein sources, rather than plant-based sources (Figure 10c). A detrimental effect of animal protein was only observed in North America and Europe, but not in Japan(Reference Chen, Glisic and Song261), suggesting that pathways linking animal protein and CVD mortality might be related to nutrients (e.g. saturated fats) other than protein content that are present in animal foods. For instance, fish was associated with reduced CVD mortality in observational studies(Reference Chareonrungrueangchai, Wongkawinwoot, Anothaisintawee and Reutrakul8). For legumes, which are rich not only in protein but also in fibre, a review of observational studies showed associations between intake and reduced coronary heart disease risk and CVD mortality, but not stroke(Reference Chareonrungrueangchai, Wongkawinwoot, Anothaisintawee and Reutrakul8). In terms of dairy, a recent overview of systematic reviews showed that, in observational studies, consumption of total dairy was associated with risk of stroke, but not consistently associated with CVD, coronary heart disease or myocardial infarction risk. When looking at specific dairy foods, yoghurt or buttermilk were not significantly associated with CVD, coronary heart disease or stroke, while fermented milk showed benefits in terms of CVD, coronary heart disease and stroke incidence and mortality, and milk and cheese showed mixed findings for all CVD outcomes(Reference Fontecha, Calvo, Juarez, Gil and Martínez-Vizcaino262), thus highlighting that particular fermented dairy products might be of relevance.
Other foods, such as fruits and vegetables, were associated with decreased risk of stroke, but did not decrease the risk of coronary heart disease and CVD mortality(Reference Chareonrungrueangchai, Wongkawinwoot, Anothaisintawee and Reutrakul8), which might potentially be linked to the fibre and specific minerals content. Cacao, coffee and green tea are recognised for their phytochemical content, which is hypothesised to exert CVD protection. In systematic reviews of observational studies, chocolate reduced coronary heart disease, stroke and CVD mortality, and coffee was associated only with CVD mortality and green tea with reduced risk of stroke(Reference Chareonrungrueangchai, Wongkawinwoot, Anothaisintawee and Reutrakul8). In addition, we found emerging evidence from a systematic review of cross-sectional and prospective studies demonstrating that a high intake of ultra-processed foods is associated with increased risk of CVD and cerebrovascular disease(Reference Pagliai, Dinu, Madarena, Bonaccio, Iacoviello and Sofi263).
Dietary-pattern approach
To account for the cumulative effect of and interactions between nutrients and foods on health outcomes(Reference Hu264), analysis of the impact of dietary patterns on CVD is becoming more common in nutrition research (Figure 10d). In systematic reviews of RCTs, consumption of healthy diets, classified using the Healthy Eating Index, for example, has resulted in reduced CVD risk in high-risk populations, yet no benefits in CVD mortality for the general population were observed(Reference Chareonrungrueangchai, Wongkawinwoot, Anothaisintawee and Reutrakul8). In addition, the Mediterranean diet, which is composed mostly of fruits, vegetables, whole grains, legumes and beans, olive oil and nuts, with moderate amounts of animal proteins, resulted in reduced risk of myocardial infarction, stroke and CVD incidence/mortality in RCTs, with mixed findings on coronary heart disease(Reference Chareonrungrueangchai, Wongkawinwoot, Anothaisintawee and Reutrakul8,Reference Dinu, Pagliai, Casini and Sofi265) . Associations with carotid intima–media thickness, which is an established surrogate marker of preclinical atherosclerosis, still need to be confirmed, as discussed in a systematic review of cross-sectional studies and RCTs(Reference Bhat, Mocciaro and Ray266). Another dietary pattern that also showed promising results in terms of CVD prevention is the Dietary Approaches to Stop Hypertension (DASH). Similar to the Mediterranean diet, the DASH diet is also focused on fruits and vegetables, and whole grains. Protein sources include low-fat dairy, fish, poultry and nuts, while red meat is limited. DASH also reinforces foods that are high in potassium, calcium and magnesium and low in sodium. An umbrella review of prospective studies showed that consumption of the DASH diet decreased the incidence of cardiovascular disease, coronary heart disease and stroke(Reference Chareonrungrueangchai, Wongkawinwoot, Anothaisintawee and Reutrakul8). The DASH diet was also associated with reductions in carotid media thickness(Reference Bhat, Mocciaro and Ray266). Comparisons between the DASH diet and the Mediterranean diet show that the DASH diet is more effective in reducing blood pressure, with more pronounced benefits observed among individuals with larger reductions in body weight(Reference Schwingshackl, Chaimani and Schwedhelm267). The Mediterranean diet, on the other hand, has less consistent evidence in terms of systolic blood pressure, diastolic blood pressure, triacylglycerols, HDL-cholesterol, insulin, HbAc1 and LDL-C, but more consistent results in terms of body weight/fat, total cholesterol, glucose and C-reactive protein(Reference Dinu, Pagliai, Casini and Sofi265). Ultimately, adherence to the Japanese diet consisting of eight components (high intake of rice, miso soup, seaweeds, pickles, green and yellow vegetables, fish, and green tea; low intake of beef and pork) has also been associated with a decreased risk of CVD mortality among adults living in multiple areas across Japan(Reference Matsuyama, Sawada and Tomata268). To date, the Palaeolithic diet(Reference Ghaedi, Mohammadi and Mohammadi269) and Nordic diet(Reference Ramezani-Jolfaie, Mohammadi and Salehi-Abargouei270) have been associated with physiological risk factors for CVD, but not CVD clinical endpoints.
In addition to the more comprehensive dietary patterns which provide recommendations and targets for various food groups, there are diets that focus on the exclusion of specific food groups, such as the low(er) carbohydrate or vegetarian diets. A systematic review of observational studies showed that high adherence to a vegetarian diet was associated with reduced coronary heart disease, with no reported impact on stroke or CVD mortality(Reference Chareonrungrueangchai, Wongkawinwoot, Anothaisintawee and Reutrakul8). In similar studies, low-carbohydrate diets were not associated with reductions in risk of CVD incidence or mortality(Reference Noto, Goto, Tsujimoto and Noda254), yet RCTs reported improvements in traditional risk factors for CVD(Reference Goldenberg, Day and Brinkworth271). In diets that are focused on the exclusion of particular food groups, careful consideration of replacement or substitute food groups is required. For instance, a meta-analysis of prospective studies showed that low-carbohydrate diets were associated with increased mortality when carbohydrates were exchanged for animal-derived fat or protein, and decreased when the substitutions were plant-based fats or proteins(Reference Seidelmann, Claggett and Cheng272). Low-carbohydrate diets can also result in reductions or elimination of whole grains or fibres, which, as previously described, have shown cardioprotective benefits.
From the evidence presented, we tentatively conclude that different dietary factors impact CVD risk via different pathways. Diets should be considered in the context of the nutrients they encompass, and interventions to address CVD risk should be seen in the context of individuals/populations being treated, which have implications for both research and practice.
Innovation in future research
As RCTs are difficult to perform, time consuming and not meant to answer all questions regarding the role of diet in CVD incidence and mortality, epidemiological research must not only recognise that different dietary patterns impact CVD risk via different nutrients and physiological pathways but also to pursue innovative efforts to properly address the complexities that are inherent to both domains.
A new research direction in nutrition science includes the use of causal inference thinking and analytical methods (e.g. matching, inverse probability, G-estimation). Three key conditions are required to enable causal inferences to be made from observational data: consistency (i.e. the values of treatment under comparison correspond to well-defined interventions), exchangeability (i.e. probability of receiving every value of treatment depends only on measured covariates) and positivity (i.e. the probability of receiving every value of treatment is greater than zero)(Reference Hernán and Robins273). Causal inference thinking and analysis aim to emulate a randomised experiment and extract evidence for cause-and-effect relationships by investigating well-defined interventions(Reference Hernán and Taubman274) and counterfactual scenarios from observational studies(Reference Splawa-Neyman275–Reference Imbens278). For example, in a causal inference study, researchers pose questions such as ‘what is the impact of diet X on cardiovascular risk Y?’ and draw structural relationships between exposures and outcomes, taking account of confounders and mediators. Subsequently, observational data are used to corroborate or invalidate these relationships. Unlike standard statistical approaches, causal inference analysis allows not only the pathways to be inferred from the data but also the nature of the functional relationships among risk drivers and outcome variables. These techniques address a common problem in epidemiological research where highly nonlinear relationships cannot be captured properly with standard statistical approaches that typically assume linear or log-linear relationships and make strong assumptions with respect to the behaviour of residuals.
Considering the complexity of biological processes and their interactions with built and social environments, substantial challenges remain before we can robustly establish cause and effect from observational data. Expert knowledge of the causal structure underlying diet and health issues remains a crucial component in causal inference analysis and interpretation(Reference Hernán, Hsu and Healy279). The diagrams included in this paper present an alternative way to consolidate and better visualise available evidence. For instance, the diagrams provide information on the type of evidence supporting the link between a number of variables and CVD outcomes, thus highlighting which of these are potential risk factors for CVD (e.g. consistent evidence from randomised studies). Although the diagrams are currently presented as separate figures, future developments include their integration into an interactive platform that would enable researchers and clinicians to appreciate the complexity of questions linking diet and CVD outcomes and better design causal diagrams, which are considered useful tools for identifying, measuring and addressing potential confounders(Reference Glass, Goodman, Hernán and Samet280). Future developments in this area also include the use of more extensive datasets.
In addition, the area of precision nutrition also deserves further exploration as individuals’ responses to diets may vary based on their phenotype or ‘metabotype’ (which has a multitude of determinants not fully understood yet)(Reference Palmnäs, Brunius and Shi281). One large prospective study, followed by a randomised trial, showed that the use of an algorithm that integrated blood parameters, dietary habits, anthropometrics, physical activity and gut microbiota information accurately predicted and improved individuals’ glycaemic responses to a nutrition intervention(Reference Zeevi, Korem and Zmora282). Phenotypic factors that influence response to nutrition interventions remain largely unexplored, and studying them could further improve our understanding of nutrition intervention results(Reference Gibney283).
Implications for evidence-informed practice
Current clinical practice addressing cardiovascular disease blends the interaction between population research with the individual the clinician is caring for. Managing CVD risk begins with modifying lifestyle factors, followed by introducing medication for treating hypertension, diabetes mellitus type 2 and hypercholesterolaemia. However, guidelines on how to help patients change their lifestyle are lacking for medical professionals, who often rely on the use of knowledge from very limited training in medical schools or their own research(Reference Macaninch, Buckner and Amin284).
One reason guidelines may be lacking for clinicians to give advice on lifestyle change is the recognition that generic advice which is effective and safe is not often possible for the whole population. In terms of diet, consideration of the trade-offs in terms of risks/benefits must be considered in the context of individual CVD risk profiles, along with factors such as accessibility and long-term feasibility. As such, clinicians must identify and tailor advice to achieve success. However, with the dawn of precision nutritional care, algorithms or guidelines for specific sub-sets of the population may be possible to create, allowing clinicians to feel more comfortable initiating lifestyle changes tailored to the individual. One challenge is that precision medicine is difficult to deliver in primary care. Another challenge lies in doing more extensive and more rigorous research to create robust algorithms to support effective personalised care using objective health risk scoring methods that seamlessly incorporate diet- and lifestyle-related risk factors amongst others. This would entail using blood samples from hundreds of thousands of participants to create a dataset that can be compared and ultra-fast computers to process these data in depth, enabling the development of algorithms that are more adaptable and effective for each individual. Dealing with confounders becomes even more important when developing guidelines for personalised interventions.
Conclusion
Current evidence from observational studies regarding the role of blood pressure disturbances suggests that less understood disorders such as white-coat hypertension, masked hypertension (normal blood pressure reading in the clinic but elevated during ambulatory measurements), orthostatic hypertension and pre-eclampsia are not completely benign in terms of CVD risk. In experimental studies, not all groups of hypertensive patients and not all CVD outcomes benefited from intensive reductions in blood pressure. Similarly, several markers of disturbances in glucose and insulin metabolism have been associated with increased risk of particular CVD events and/or mortality. Early stages of diabetes, including gestational diabetes, were also linked with increased CVD events, potentially due to increased risk to develop type 2 diabetes in the long term. Reducing glucose levels below standard targets showed no benefits for patients with type 2 diabetes and high risk of CVD, yet studies in the primary prevention context are still needed. Although conventional lipid markers such as LDL-C, HDL-C and TGL have been largely associated with overall CVD risk, for particular CVD outcomes, such as ischaemic stroke, the evidence is less consistent. More recent evidence in this domain points to the relevance of various apolipoproteins, and of more specific components, such as proteins bound to cholesterol or cholesterol sub-particles. Different from intensive reductions on glucose levels, reducing LDL-C below standard targets was associated with increased reductions in CVD events among high-risk patients, at the expense of increased risk of intracerebral haemorrhage. For obesity, different markers that suggest accumulation of adipose tissue, as well as substances secreted by adipocytes, were associated with increased CVD events and a few of them with increased CVD mortality. RCTs of pharmacologically induced weight loss demonstrated reduced CVD mortality, yet evidence of other approaches such as low-fat diets and bariatric surgery is still limited. Although there are several emerging markers of disturbances in blood pressure, glucose and insulin, lipids and fat deposition, the utility of including such markers in CVD prediction tools or targeting them in CVD prevention therapies remains unknown. Also, the heterogeneity in the benefit of targets above the usual standards across different groups of patients suggests that future studies should be sensitive to the background risk of patients and the relevance of other factors in CVD risk. Ultimately, future studies should also consider the specific mechanisms of different CVD diseases, as both established and new markers of blood pressure, glucose and insulin metabolism, lipid metabolism and fat deposition were associated with some CVD outcomes but not others.
Evidence regarding the role of more emerging risk factors, such as inflammatory conditions, relies mostly on observational studies that highlight different inflammatory diseases and markers associated with increased CVD risk, yet more studies are still required to determine causal relationships and specific targets for treatment. On the contrary, there are several experimental studies showing benefits of anti-coagulation therapies for CVD, yet many coagulation markers still need to be explored to determine causality. Ultimately, evidence regarding the role of endothelial dysfunction, which has been considered an important mediator between other risk factors and CVD events, also relies on observational studies, and further exploration is still required.
This review also highlighted additional factors that might influence CVD risk through the previously discussed physiological risk factors, including pre-birth and early childhood conditions, yet evidence also relies on observational studies which are subject to several confounders. Intervention studies were scarce, potentially due to long-term follow-up required to observe the impact on CVD clinical outcomes. Although not extensively, we also presented additional factors such as those related to the environment which might influence CVD risk, such as air pollution, socio-economic deprivation and diet in particular.
In terms of diet, many aspects might impact cardiovascular health. Although network analysis suggests superiority of particular dietary patterns over others, which might be related to the cumulative effect of various nutrients on different risk factors for CVD, different results still present for different individuals, irrespective of adherence issues. To move forward and disentangle the effect of diet on CVD, the scientific community should consider the multifactorial nature of CVD and the complex interplay between physiological conventional and emerging risk factors with natural and built environments, while bringing the life course into the spotlight. To bring together the different confounders that would account for biased estimates in observational studies, nutrition research would benefit from large collaborations and virtual tools that organise information and data across the different pathways that lead to CVD. Experimental research would benefit from exploring the impact of diet on physiological and conventional to novel and emerging risk factors linked to CVD as part of a continuum, where the interplay between individual risk factors needs to be better understood and, where possible, quantified. Whilst the challenge in translating results from controlled environments to real-life contexts remains, further research strategies are needed to systematically harness routine ‘real-world’ data such as those from electronic health records and take a causal inference analysis approach to investigating temporal and aetiological factors highlighting previously underutilised opportunities for risk mitigation, particularly through the modulation of diet and lifestyle factors.
Acknowledgements
We would like to acknowledge Mary Ward and Luigi Palla for commenting on the relevance of this manuscript and Nate Jensen for contributing to the broader Causal Inference Project.
Financial support
The Swiss Re Institute and Core Funding to the NNEdPro Nutrition Research and Innovation Consortium.
Conflict of interest
No conflicts of interest to declare.
Authorship
M.R.L.V. was responsible for the project administration, as well as conceptualisation, data curation and analysis and writing the original draft of the manuscript. L.B., C.G.M., C.R.T., S.K.K., A.K. and S.A. contributed to the conceptualisation, data curation and analysis, and writing the original draft of the manuscript. S.M. and X.D. contributed to writing the original draft of the manuscript. R.G., D.U., J.B. and L.G. contributed to reviews and editing. S.R. and R.G. were responsible for the supervision, review and edits, and funding acquisition.