Introduction
Dietary protein provides the body with amino acids as the building blocks for protein synthesis, but dietary amino acids are also utilised catabolically, and perform important signalling and metabolic roles in the gut and throughout the body(Reference Trommelen, Tomé and van Loon1). The rate of protein hydrolysis in the gut after a meal determines the time course of local peptide and amino acid concentrations along the gastrointestinal lumen(Reference Montoya, Cabrera and Zou2). Local concentrations of amino acids and peptides in turn trigger metabolically important processes in the gut, such as enteroendocrine functions(Reference Mace, Schindler and Patel3).
A high-protein meal elevates plasma amino acid concentrations for several hours, and the rate of amino acid absorption determines the maximum concentration (Cmax), duration and area-under-the-curve (AUC) of the post-prandial amino acid concentration time courses. Plasma amino acid concentrations control the balance between catabolic and anabolic utilisation of amino acids in many tissues(Reference Fouillet, Juillet and Gaudichon4) and have an important influence on muscle protein synthesis(Reference Pinckaers, Trommelen and Snijders5,Reference West, Burd and Coffey6) and energy homeostasis(Reference Schwartz7). The rate of dietary protein digestion and absorption thus has wide-ranging metabolic effects.
Considerable effort has been devoted to studying the bioavailability of “fast” and “slow” proteins, the most notable examples of which are whey proteins (“fast”) and caseins (“slow”) from milk(Reference Gorissen, Trommelen and Kouw8). Native whey proteins do not coagulate under gastric conditions(Reference Peram, Loveday and Ye9), and therefore empty rapidly from the stomach, whereas gastric-coagulating caseins empty more slowly(Reference Ye10). Research has highlighted the key role of gastric emptying rates in controlling the kinetics of proteolysis and absorption(Reference Huppertz and Chia11–Reference Velchik, Reynolds and Alavi13).
Food processing is known to affect the overall bioavailability of protein(Reference Gerrard, Lasse and Cottam14), but what is less well known is that food processing can alter the rate of protein digestion and absorption, i.e. processing can make a given protein source “faster” or “slower”. I review evidence that food processing affects the kinetics of amino acid release and absorption following a protein-rich meal, and discuss the physicochemical phenomena responsible. I focus on studies that used test meals processed in different ways, but with near-identical composition. The evidence suggests that food processing can be an effective tool to optimise protein digestion and absorption for desired metabolic outcomes.
The scope of this review is limited to in vivo trials on human volunteers or pigs, which are considered a gold standard animal model of human digestion(15). The experimental details of studies discussed here are summarised in Table 1.
Table 1. Overview of studies discussed in the main text.
Protein digestion and absorption
As protein digestion and absorption processes in the human body have been reviewed in detail by others(Reference Trommelen, Tomé and van Loon1,Reference Mackie and Macierzanka16) , I provide only a brief overview here. I use the term digestion to refer to the breakdown of protein into peptides and amino acids, and absorption to refer to the uptake of breakdown products from the gastrointestinal lumen by enterocytes.
Protein digestion begins in the mouth, where food is disrupted by chewing and mixed with saliva, then swallowed. In the stomach, the actions of acid and pepsin, combined with peristaltic mixing, further break down food and initiate proteolysis. Gastric pH typically rises after a meal due to the buffering effect of food materials, and subsequently decreases due to gastric secretions. Pepsin is most active at pH ∼2, but it shows partial activity up to pH ∼6, so the inhibition of pepsin activity by post-prandial buffering of gastric pH may be relatively short-lived(Reference Salelles, Floury and Le Feunteun17). Proteolysis in the stomach is limited, serving mainly to release peptides and aromatic amino acids that alert gut sensing systems to the composition of the meal(Reference Moran, Daly and Al-Rammahi18).
The emptying of stomach contents into the duodenum is influenced by the physical properties of gastric digesta, i.e. foods that coagulate or show high viscosity under gastric conditions empty more slowly than liquid foods(Reference Huppertz and Chia11,Reference Jin, Wilde and Hou12) . A higher energy content of a meal also slows gastric emptying(Reference Velchik, Reynolds and Alavi13).
In the duodenum, bile and pancreatic enzymes are added to the gastric chyme, as well as bicarbonate, to bring pH close to neutral. Further secretions of bicarbonate, water and mucus are added in the jejunum and ileum(Reference Le Feunteun, Al-Razaz and Dekker19). During transit through the ileum, proteases in the lumen cleave dietary protein into short peptides and amino acids. Physiological surfactants such as bile acids and phospholipids also contribute to proteolysis by denaturing proteins, which renders them more susceptible to proteases(Reference Mackie and Macierzanka16). The villi on the apical surface of enterocytes (the “brush border”) contain anchored proteases, but also secrete protease-containing vesicles into the periapical space(Reference Hooton, Lentle and Monro20).
Some peptides and amino acids are thought to exert bioactivities through interactions with receptors in the gastrointestinal tract(Reference Fernández-Tomé and Hernández-Ledesma21,Reference Moughan, Fuller and Han22) . Ex vivo studies with animal tissue have shown that casein-derived peptides can stimulate water absorption(Reference Mansour, Tomé and Rautureau23) and decrease intestinal mobility(Reference Dalziel, Spencer and Dunstan24). A recent study with human intestinal biopsy tissue showed that a soybean peptide had immunomodulatory activity(Reference Fernández-Tomé, Indiano-Romacho and Mora-Gutiérrez25). At present, there is no direct evidence of diet-derived exogenous peptides exerting bioactivities in humans, but the aforementioned studies and others suggest that some food-derived peptides may be bioactive in the gut. Exogenous bioactive peptides have been identified in human jejunal aspirates(Reference Boutrou, Gaudichon and Dupont26), and the effect of protein structure on the kinetics of digestion can alter the time course of peptide and amino acids released into the intestinal lumen(Reference Barbé, Le Feunteun and Rémond27). However, the effect of protein digestion rates on peptide bioactivities in vivo has not been reported yet.
Dietary protein is not the only source of amino acids in the intestinal lumen; endogenous proteins are secreted into the gut in the form of mucus, enzymes, sloughed off cells, etc. Daily ileal endogenous nitrogen loss has been estimated at 2026±441 mg/d(Reference Deglaire, Moughan and Airinei28). The amino acids of endogenous proteins are partly recycled through digestion, and endogenous proteins may be an important source of bioactive peptides(Reference Dave, Hayes and Montoya29).
Amino acids, di- and tri-peptides are taken up from the intestinal ileum into enterocytes through various apical amino acid transporters, and released into the portal vein blood via different basolateral transporters(Reference Bröer and Gauthier-Coles30). The splanchnic tissues use amino acids for protein synthesis and energy, and retention rates differ by amino acid(Reference Bröer and Bröer31). Overall splanchnic retention of exogenous nitrogen is 29–60 %(Reference Engelen, Rutten and De Castro32–Reference Groen, Horstman and Hamer35), and may be higher for the elderly than for adults(Reference Moreau, Walrand and Boirie36). The unused amino acids and peptides are released into circulation.
The true ileal digestibility is the proportion of ingested protein that disappears before the terminal ileum (corrected for endogenous losses), and this proportion is assumed to have been absorbed(Reference Trommelen, Tomé and van Loon1). True ileal amino acid digestibility values of >80% are common for amino acids in food proteins(Reference Moughan, Gilani and Rutherfurd37), but a small proportion of protein or peptides may pass into the large intestine, particularly if digesta viscosity is enhanced by dietary fibre(Reference Gidley38). The gut microbiota can hydrolyse protein and metabolise amino acids, and colonocytes may be capable of limited amino acid absorption(Reference van der Wielen, Moughan and Mensink39).
A high-protein meal produces post-prandial increases in plasma free amino acid concentrations that can last for several hours. Peripheral blood sampling remains the simplest and least invasive way to track dietary protein absorption, but results must be interpreted with caution because plasma amino acid concentrations reflect a multitude of dynamic processes (Figure 1)(Reference Trommelen, Tomé and van Loon1). Protein synthesis leads to removal of amino acids from the blood stream, and tissue breakdown adds amino acids into the blood stream(Reference Waterlow40). Dispensable amino acids in blood partly reflect biosynthesis rates, and various tissues remove circulating amino acids to oxidise them for energy(Reference Bröer and Gauthier-Coles30).
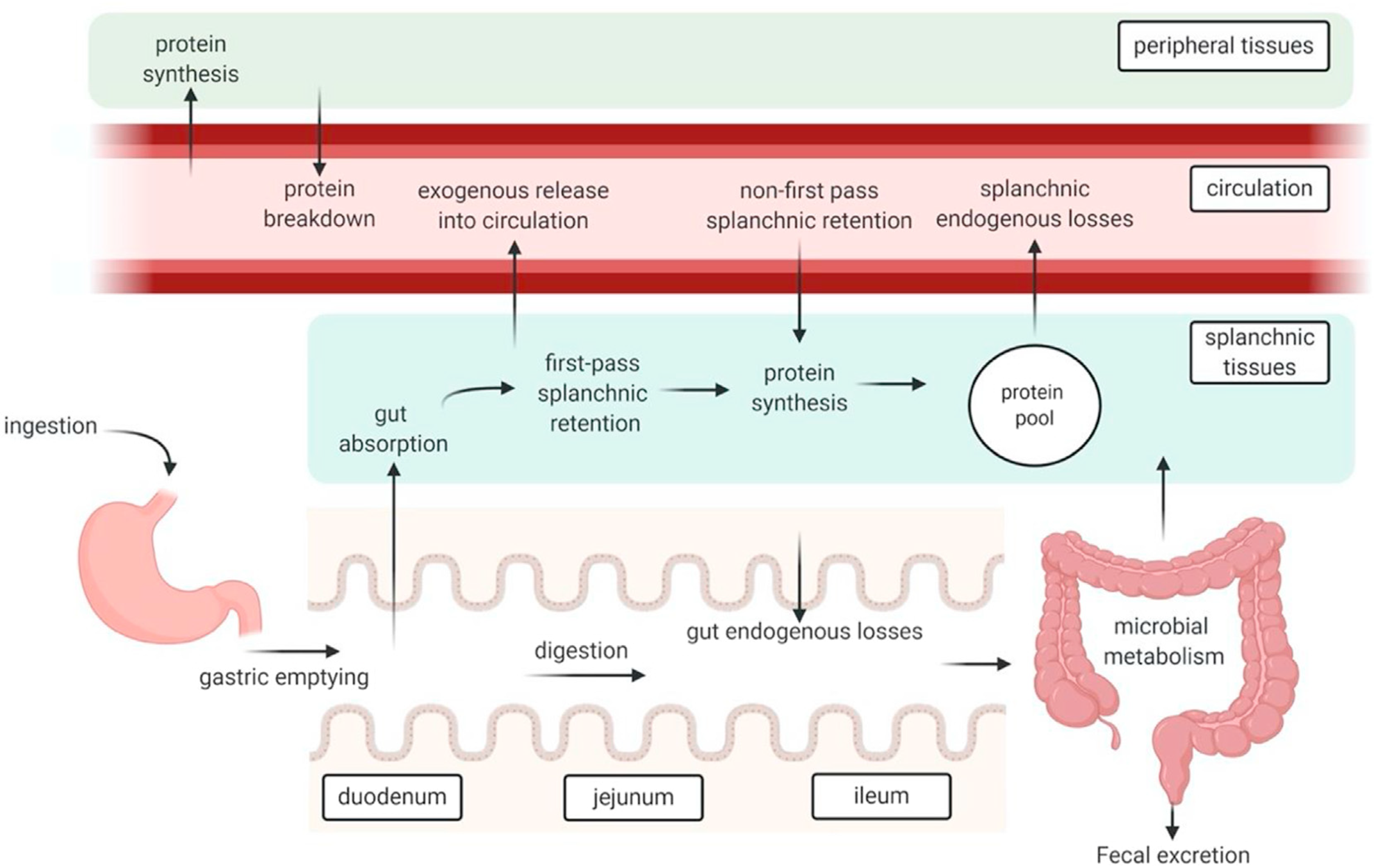
Fig. 1. Schematic representation of protein digestion and absorption processes. After Trommelen et al. (Reference Trommelen, Tomé and van Loon1), reproduced with permission.
The relative rates of protein synthesis, protein breakdown and amino acid oxidation contribute to free amino acid homeostasis(Reference Bröer and Bröer31,Reference Waterlow40) , which is under tight hormonal and central nervous system control. Insulin stimulates amino acid uptake into muscle for tissue synthesis(Reference Fujita, Rasmussen and Cadenas41), but amino acids stimulate insulin release in the pancreas(Reference Bröer and Bröer31), so amino acid homeostasis is intrinsically linked to glucose homeostasis.
After a high-protein meal, the rate of amino acid influx into the intestinal lumen, splanchnic tissue and circulating free amino acid pool determines the magnitude and direction of change among protein synthesis, protein breakdown and amino acid oxidation processes(Reference Waterlow40). Protein digestion and absorption also influence other processes via the signalling roles of certain free amino acids. For example, K-cells in the intestinal epithelium release glucose-dependent insulinotropic polypeptide (GIP) in response to increased concentrations of free amino acids and dipeptides in the intestinal lumen(Reference Reimann, Diakogiannaki and Moss42). The hypothalamus senses the post-prandial increase in blood leucine concentration and suppresses glycogenolysis and gluconeogenesis(Reference Schwartz7).
Here I discuss evidence that food processing modulates protein digestion and absorption rates via effects on food microstructure. Trials with food ingredients and formulated foods are discussed separately from wholefood trials because natural food microstructures and heterogenous composition in wholefoods introduce extra complexity into digestion processes. The wider metabolic effects of differential protein digestion rates are not well understood, but present in vivo results suggest that rational control of protein digestion and absorption by careful selection of food processing conditions could be a useful tool to optimise metabolic response to protein-rich meals.
Food ingredients and formulated foods
Process-induced amino acid side chain modifications
Food processing can involve extremes of temperature and pH. Under these conditions some amino acid sidechains are susceptible to undergoing chemical reactions(Reference Gerrard, Lasse and Cottam14,Reference McKerchar, Clerens and Dobson43) that cause permanent loss of bioavailability(Reference Moughan and Rutherfurd44). Amino acid side chains can undergo acid-/alkali- and water-catalysed reactions such as oxidation and deamination, or can react with other amino acids, leading to non-native cross-links(Reference McKerchar, Clerens and Dobson43). Side chains can react with other food components, for example Maillard reactions with reducing sugars, leading to glycation and further reactions(Reference Al Jahdali and Carbonero45).
The indispensable amino acids histidine, lysine, methionine, threonine and tryptophan are particularly reactive, and reactions catalysed by heat, oxidising conditions or alkaline pH will compromise their bioavailability(Reference Gilani, Xiao and Cockell46,Reference Rutherfurd, Montoya and Moughan47) and may give rise to toxic derivatives(Reference Gilani, Xiao and Cockell46,Reference Kolpin and Hellwig48) . Special precautions are required when analysing lysine in processed food or feed, because Maillard-reacted lysine (which is not bioavailable) reverts to lysine during the acid hydrolysis step in amino acid analysis(Reference Rutherfurd49).
Nyakayiru et al. (Reference Nyakayiru, Van Lieshout and Trommelen50) measured the effect of glycation on lysine peripheral bioavailability with milk protein powders that had minimal glycation (3%), or glycation of 20% or 50% after dry heating at 50°C. In 15 healthy volunteers, glycation of 20% and 50% reduced post-prandial plasma lysine iAUC (incremental AUC) by 35% and 92%, respectively (Figure 2). Although the level of lysine glycation was artificially manipulated in this study, site-specific glycation rates of ≥20% have been observed in commercial milk products whose manufacture involves severe heating(Reference Meltretter, Wüst and Pischetsrieder51), and this is thought to be responsible for reduced lysine bioavailability(Reference Rutherfurd and Moughan52).
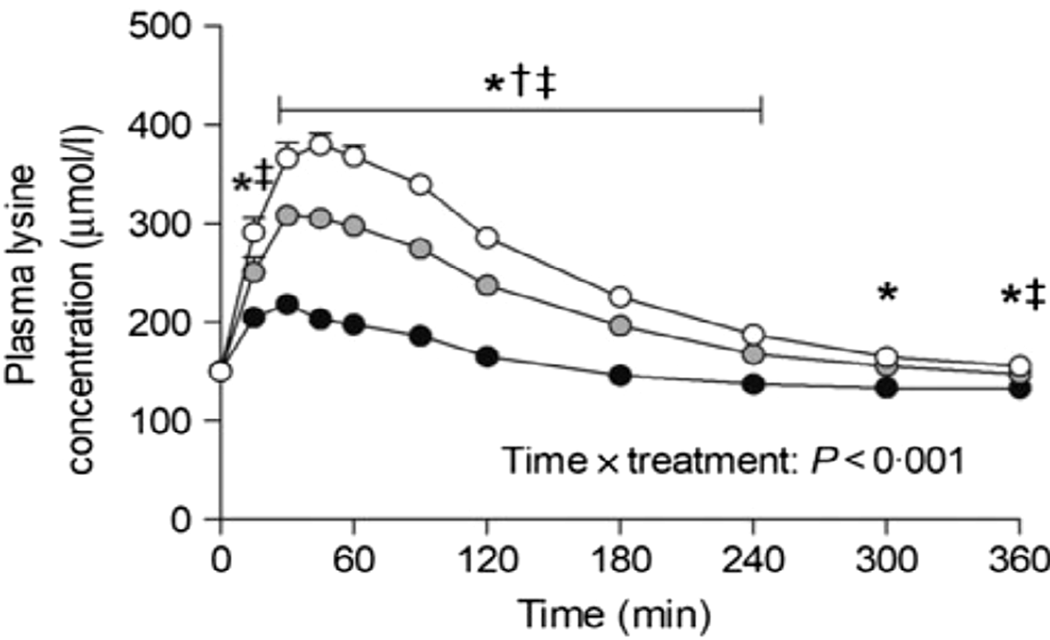
Fig. 2. Post-prandial plasma lysine concentrations for volunteers consuming milk protein powders with lysine glycation of 3% (open circles), 20% (grey circles) or 50% (black circles). After Nyakayiru et al. (Reference Nyakayiru, Van Lieshout and Trommelen50), reproduced with permission. *Significantly lower concentrations following ingestion of 50% glycation than 3% glycation (P<0·001). †Significantly lower concentrations following ingestion of 20% glycation than 3% glycation (P≤0·029). ‡Significantly lower concentrations following ingestion of 50% glycation than 20% glycation (P<0·001).
The effect of Maillard reactions on lysine bioavailability was also seen by Rérat et al. (Reference Rérat, Calmes and Vaissade53) in pigs after feeding heat-treated skim milk powder (51% lysine blockage). The cumulative appearance of lysine in portal blood was reduced by 60% relative to unheated skim milk powder. Valine absorption was also reduced (−34%) by heat treatment, but cystine absorption increased (+37%). Other in vitro and in vivo studies of processing-induced amino acid modifications to dairy proteins were reviewed by van Lieshout et al. (Reference van Lieshout, Lambers and Bragt54).
Relatively little is known about process-induced amino acid modifications in foods other than dairy products, partly due to the diversity of reaction products and the complexity of mass spectrometric analysis. In a survey of commercial soybean meal ingredients, Troise et al. (Reference Troise, Wiltafsky and Fogliano55) reported that 26 out of 80 samples had ≥20% blocked lysine. Lassé et al. (Reference Lassé, Deb-Choudhury and Haines56) reported that boiling egg white protein created a wide range of oxidative and other amino acid modifications. In blanched navy beans, Deb-Choudhury et al. (Reference Deb-Choudhury, Cooney and Brewster57) found lysinoalanine cross-links and heat-induced amino acid modifications.
Early Maillard reaction products appear to have low digestibility, but they are absorbed to some extent and excreted in urine(Reference Rérat, Calmes and Vaissade53,Reference Somoza, Wenzel and Weiß58) . Low digestibility may derive from the inability of trypsin to recognise lysine-adjacent cleavage sites (according to Keil rules(Reference Rodriguez, Gupta and Smith59)) when lysine is glycated, or digestive enzymes may be sterically hindered from accessing cleavage sites near glycated and/or cross-linked lysine(Reference van Lieshout, Lambers and Bragt54). Alkali exposure induces cross-linking of certain amino acids with the ϵ-NH2 group of lysine via elimination–addition reactions(Reference Friedman60), as well as amino acid racemisation(Reference Friedman60). Alkali-induced cross-links may similarly obstruct digestive enzymes, and lysinoalanine does not appear to be absorbed in rats(Reference Somoza, Wenzel and Weiß58) or humans(Reference Erbersdobler, Lohmann and Buhl61), but can be absorbed by ruminants(Reference Friedman and Brandon62). D-amino acids are utilised by humans to different extents, but D-lysine is not utilised(Reference Friedman63).
Collectively, these data suggest that certain food processes involving extreme heating in the presence of reducing sugars and/or alkaline pH can modify amino acid side chains in ways that impair bioavailability. In contrast, more moderate heat treatments can improve protein digestibility through a combination of inactivating enzyme inhibitors(Reference Gilani, Xiao and Cockell46) and denaturing proteins so that they are more susceptible to enzymatic cleavage(Reference Salazar-Villanea, Hendriks and Bruininx64).
Aggregation and gelation
Many food proteins will gel under certain conditions, i.e. they will aggregate into supramolecular protein–protein networks that percolate across an entire sample and give it a self-supporting semi-solid form. Familiar foods such as cheese, yoghurt, gelatin jelly and tofu are examples in which proteins are responsible for the gelled texture.
A series of studies by a group of French scientists(Reference Barbé, Ménard and Le Gouar65–Reference Dupont, Le Feunteun and Marze68) illustrated the magnitude of gelation effects on plasma amino acid kinetics using a pig model of human digestion. In these studies, heated milk (90°C 10 min) was gelled with rennet (cheese-like texture), gelled with acid (set yoghurt texture), or gelled with acid and then stirred (stirred yoghurt texture).The kinetics of amino acid appearance in the blood were strongly altered by gelation (Figure 3), and the different gel types produced significant differences. The effects of gelation and gel type were attributed to alteration of gastric emptying rate.
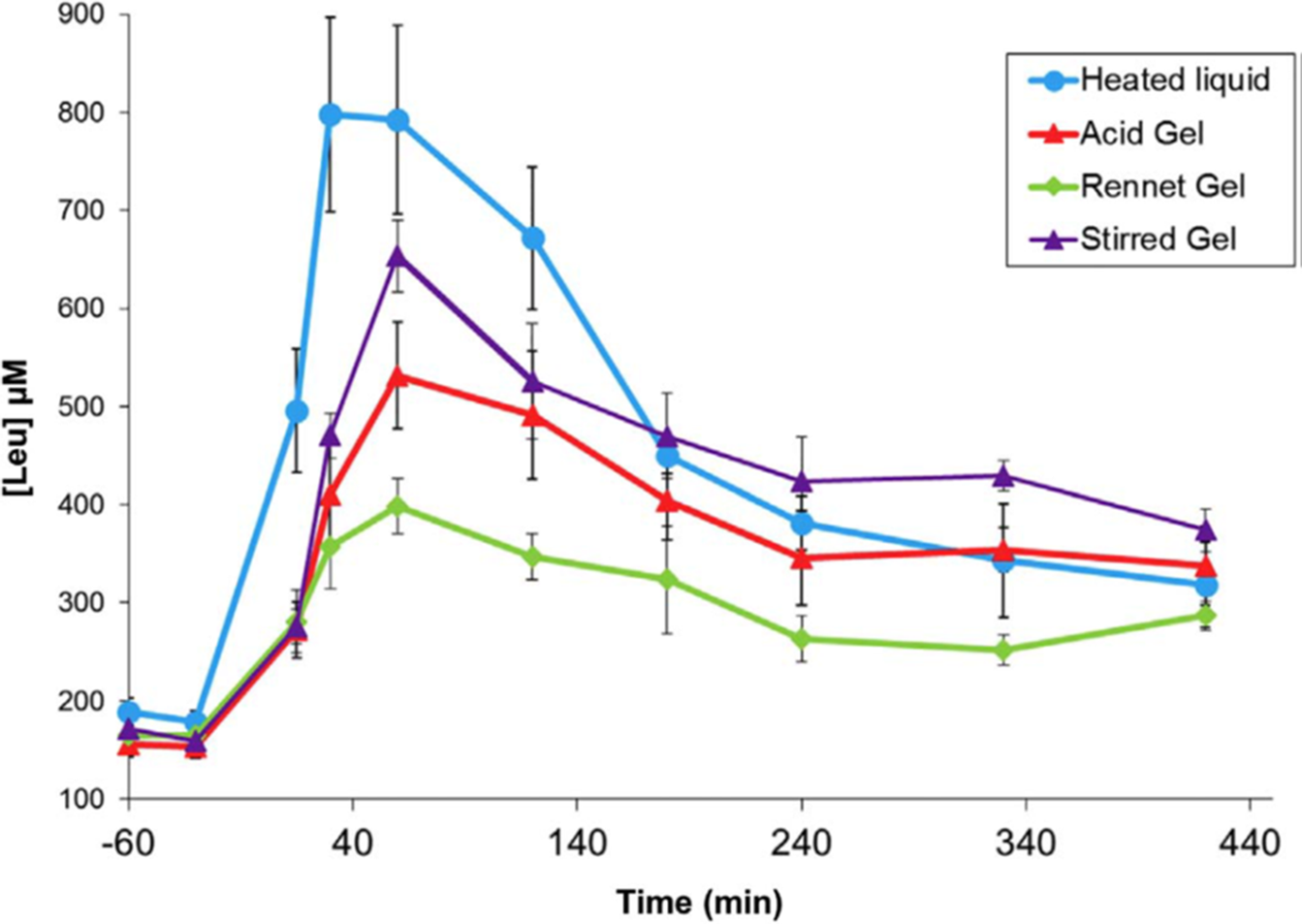
Fig. 3. Post-prandial plasma leucine kinetics in pigs after consuming milk in liquid or gelled forms. After Dupont et al. (Reference Dupont, Le Feunteun and Marze68), reproduced with permission.
Particulate or coagulated foods may retain their microstructure in the stomach, and gastric conditions may catalyse coagulation of non-particulate foods, e.g. by destabilising colloidal casein micelles(Reference Ye10). Particles or coagula that are too large to pass through the gastric pylorus are gradually eroded by the actions of pepsin and peristaltic mechanical shear. Mechanical breakup of protein coagula depends on properties such as elasticity and brittleness, and the rate of pepsin diffusion into protein coagula depends on microstructural factors such as pore size and degree of cross-linking(Reference Thévenot, Cauty and Legland69,Reference Bayrak, Mata and Raynes70) . Thus, the rate of gastric emptying is determined by the competing dynamics of microstructure formation versus mechanical/enzymatic erosion in gastric digesta. In the aforementioned studies with milk gels, gelling in different ways was thought to retard the erosion of gastric coagula to differing extents(Reference Barbé, Ménard and Le Gouar66,Reference Gaudichon, Roos and Mahe71) .
In a study with adults aged 66·7±4·3 y, Horstman et al. (Reference Horstman, Ganzevles and Kudla72) reported a small but significant difference in peak serum amino acid concentration after consuming stirred yoghurt or low-fat ultra-high temperature treated (UHT) milk. The maximum concentration of serum essential amino acids was 13% higher with yoghurt (P<0.005), but area under the curve and other parameters were not significantly different.
According to Figure 3, acid gelation of pre-heated milk as part of yoghurt making slows the digestion of milk proteins, and this effect is partially reversed by stirring, which breaks up large aggregates. The findings of Horstman et al. (Reference Horstman, Ganzevles and Kudla72) indicate that the protein in stirred yoghurt is still digested and absorbed faster than the protein in UHT milk, despite the semi-solid form of stirred yoghurt. This is probably the result of the more severe heat treatment during yoghurt manufacture (e.g. 90°C for 10 min) compared with UHT treatment (e.g. 140°C for 5 s), which results in greater whey protein denaturation(Reference Horstman, Ganzevles and Kudla72–Reference Ye, Liu and Cui74).
Some protein-rich food ingredients are deliberately aggregated as part of their manufacture, either as a way to concentrate and fractionate proteins with different properties (e.g. casein and whey) or to modify their functionality for food manufacture(Reference Ji, Fitzpatrick and Cronin75). Protein-rich food ingredients usually take the form of uniform fine powders, but with diverse microstructures that have measurable effects on protein digestion and absorption.
Trommelen et al. (Reference Trommelen, Weijzen and van Kranenburg76) measured amino acid uptake from three casein-derived food ingredients: calcium caseinate, a form of casein that has been acid precipitated and resolubilised with calcium hydroxide; micellar casein, which is produced by microfiltration of milk and retains its native micelle structure; and sodium caseinate cross-linked with the enzyme transglutaminase. The kinetics of amino acid appearance in blood plasma were similar for calcium caseinate and micellar casein, but markedly different for cross-linked sodium caseinate (Figure 4). The Cmax and iAUC for branched-chain and essential amino acids were significantly higher for cross-linked sodium caseinate.
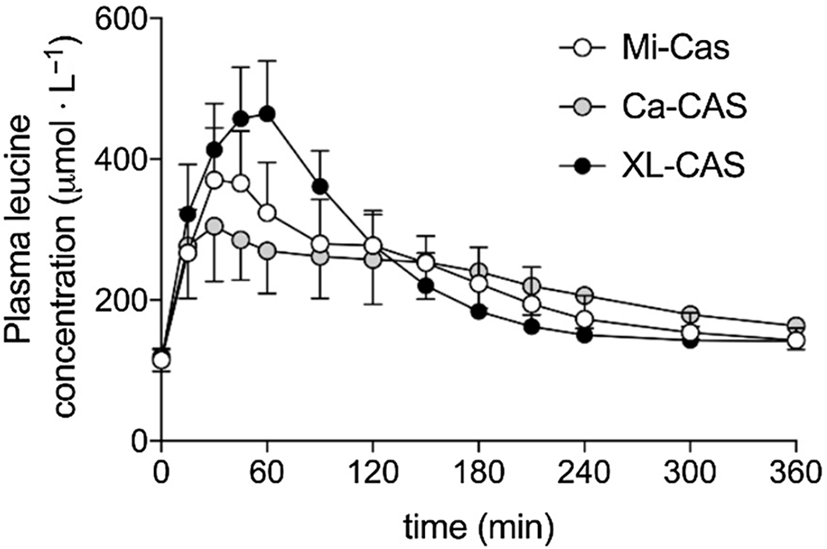
Fig. 4. Plasma leucine kinetics in volunteers after consuming different milk protein solutions. Mi-Cas, micellar casein; Ca-CAS, calcium caseinate; XL-CAS, cross-linked sodium caseinate. After Trommelen et al. (Reference Trommelen, Weijzen and van Kranenburg76), reproduced with permission.
The materials used in this study differed widely in apparent solubility after 2 h of stirring: 99%, 53% and 5% soluble for cross-linked sodium caseinate, calcium caseinate and micellar casein, respectively(Reference Trommelen, Weijzen and van Kranenburg76). In comparison with other studies(Reference Horstman, Ganzevles and Kudla72,Reference Boirie, Dangin and Gachon77) , all casein ingredients in this study gave plasma leucine time courses (Figure 4) more characteristic of whey protein than of casein. This can be understood by considering the behaviour of the three test materials under gastric conditions.
Sodium caseinate powder dissolves rapidly(Reference Ji, Fitzpatrick and Cronin75) and coagulates slowly under gastric conditions to form a loose coagulum(Reference Ye10,Reference Horstman, Ganzevles and Kudla72) . Cross-linking with transglutaminase slows down coagulation(Reference Bönisch, Heidebach and Kulozik78). The rapid absorption of transglutaminase cross-linked sodium caseinate in Figure 4 probably reflects rapid dissolution followed by emptying from the stomach before coagulation can occur.
Calcium caseinate powder dissolves slowly and incompletely: even after 2 h of stirring at 25°C most material remains as suspended particles 10–100 μm in diameter(Reference Ji, Fitzpatrick and Cronin75,Reference Moughal, Munro and Singh79) , i.e. two to three orders of magnitude larger than casein micelles(Reference Dalgleish, Boland, Golding and Singh80). Particles in this size range sediment slowly under centrifugal force, and may appear soluble. Suspended calcium caseinate particles do not coagulate under gastric conditions(Reference Horstman, Ganzevles and Kudla72), probably because of low collision frequency (high viscous drag(Reference O’Melia, Hahn and Chen81)) and/or low collision efficiency(Reference Taboada-Serrano, Chin and Yiacoumi82,Reference Salvador, Acosta and Zamora83) due to relatively large size, so they will also empty from the stomach rapidly. The overall bioavailability of the calcium caseinate in the study of Trommelen et al. (Reference Trommelen, Weijzen and van Kranenburg76) was lower than that of cross-linked sodium caseinate, which may be because a fraction of calcium caseinate particles remained partially undissolved in intestinal fluid and was therefore inaccessible to digestive enzymes.
Micellar casein powder also dissolves slowly, but ultimately dissolves completely, e.g. within ∼30 min at 25°C(Reference Ji, Fitzpatrick and Cronin75). It gave a peak in plasma leucine at 30 min, and overall bioavailability was intermediate between those of calcium caseinate and cross-linked sodium caseinate(Reference Trommelen, Weijzen and van Kranenburg76). The timing of the leucine peak suggests rapid emptying of most micellar casein from the stomach, i.e. failure to coagulate significantly under gastric conditions. The slightly higher leucine bioavailability at 150–300 min may reflect either partial coagulation in the stomach with more advanced pH decrease, or slower dissolution of this specific micellar caseinate powder compared with that tested by Ji et al. (Reference Ji, Fitzpatrick and Cronin75).
All of the materials tested by Trommelen et al. (Reference Trommelen, Weijzen and van Kranenburg76) were substantially different to native casein micelles in liquid milk, which coagulate rapidly and effectively under gastric conditions(Reference Ye10). Dissolution is a necessary precursor of coagulation due to hydrodynamic considerations. If a test meal consists of a slow-dissolving protein powder suspended (rather than dissolved) in water, then dissolution may not occur during gastric residence, and coagulation is thereby impaired.
Casein coagulation under gastric conditions is partly driven by the presence of calcium, and partial removal of calcium using zeolite treatment during casein manufacture can weaken the ability of caseins to coagulate(Reference Huppertz and Lambers84). In the work of Chan et al. (Reference Chan, D’Souza and Beals85), calcium-depleted milk protein concentrates (mMPC) gave significantly higher plasma concentrations of essential amino acids at 45–90 min post-prandially, compared with conventional MPC or calcium caseinate (Figure 5A). The MPC versus mMPC difference was attributed to weaker gastric coagulation with mMPC as a result of calcium depletion, and led to significantly higher insulin at 60 min (Figure 5B).
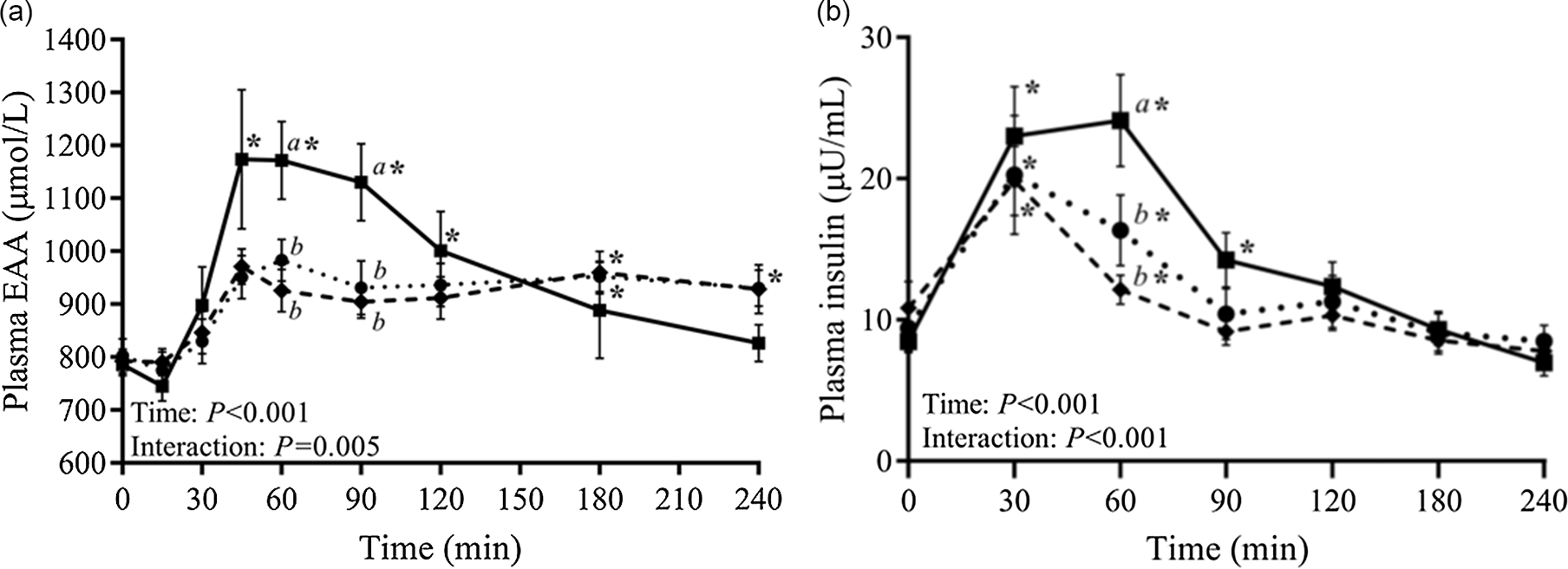
Fig. 5. Plasma concentrations of essential amino acids (EAA) (a) and plasma insulin concentration (b) after consumption of milk protein concentrate (dotted line, circles), mineral-modified milk protein concentrate (solid line, squares) or calcium caseinate (dashed line, diamonds). After Chan et al. (Reference Chan, D’Souza and Beals85), reproduced with permission.
Aggregation and/or gelation as a result of food processing alters the physical form of food proteins, and thereby affects food digestion processes. The susceptibility of protein to enzymatic proteolysis may be increased or decreased, depending on aggregate structure. Gastric coagulation slows protein digestion, and processing that affects gastric coagulation significantly impacts protein digestion rates.
In a comprehensive overview of milk protein digestion studies, Horstman and Huppertz(Reference Horstman and Huppertz86) suggested that the main factors controlling the rate of milk protein digestion are the potential of a given milk protein material to coagulate in the stomach and the energy content of a meal. Horstman and Huppertz(Reference Horstman and Huppertz86) noted that not all forms of casein will coagulate under gastric conditions. This often-overlooked nuance can have a substantial effect on plasma amino acid kinetics, as seen in the studies by Trommelen et al. (Reference Trommelen, Weijzen and van Kranenburg76) and Chan et al. (Reference Chan, D’Souza and Beals85) (Section 3.2). Homogenising milk causes casein micelles to adsorb to milk fat globule interfaces, and this substantially alters the structure and mechanical properties of coagula formed under gastric conditions(Reference Ye, Cui and Dalgleish87). It remains to be seen what the consequences of homogenisation are for milk protein digestion and absorption rates.
Hydrolysis
Food proteins can be hydrolysed by acid or enzymes prior to ingestion, and this may affect digestion and absorption kinetics, depending on the degree of hydrolysis and the presence of other food components.
Deglaire et al.(Reference Deglaire, Moughan and Airinei28,Reference Deglaire, Fromentin and Fouillet34) quantified post-prandial nitrogen flows in adult humans after consuming intact casein or casein hydrolysed with pancreatin. Ileal exogenous nitrogen flow was higher for the hydrolysed casein over the first 3 h(Reference Deglaire, Moughan and Airinei28), and this was reflected in plasma amino acid kinetics(Reference Deglaire, Fromentin and Fouillet34), particularly for indispensable amino acids (Figure 6A). Endogenous nitrogen and amino acid flows and net post-prandial protein utilisation were not significantly different for the two diets. However the hydrolysed casein diet elicited greater splanchnic retention and lower peripheral uptake, as well as significantly higher insulin production(Reference Deglaire, Fromentin and Fouillet34) (Figure 6B).

Fig. 6. Plasma amino acid (a) and plasma insulin (b) in adult humans following consumption of a meal containing intact casein (C) or hydrolysed casein (HC). After Deglaire et al. (Reference Deglaire, Fromentin and Fouillet34), reproduced with permission.
In other studies comparing intact micellar casein with commercial casein hydrolysate, Koopman et al. (Reference Koopman, Crombach and Gijsen88) and Pennings et al. (Reference Pennings, Boirie and Senden89) reported significantly higher post-prandial plasma amino acid and insulin concentrations with hydrolysates (20 g or 35 g protein bolus). Calbet and Holst(Reference Calbet and Holst90) reported similar differences in plasma amino acid kinetics with a 60 g bolus of casein or hydrolysed casein.
In the most extreme case, intact protein can be compared to a compositionally-equivalent mixture of amino acids. Weijzen et al. (Reference Weijzen, van Gassel and Kouw91) reported large and significant differences in plasma amino acid kinetics in humans after consuming milk protein (processing unspecified) or a corresponding amino acid mixture (30 g bolus). Plasma amino acid levels, insulin peak concentration and net protein balance were higher with the amino acid mixture, but muscle protein synthesis rate was not significantly different.
There are conflicting results regarding the effect of processing on whey protein digestion. Some studies have shown very similar plasma amino acid kinetics, whether native, hydrolysed(Reference Calbet and Holst90,Reference Power, Hallihan and Jakeman92) or microparticulated.(Reference Mitchell, D’Souza and Fanning93) Others have reported that intact whey protein produces higher plasma leucine than hydrolysed whey protein(Reference Farnfield, Trenerry and Carey94,Reference Hamarsland, Laahne and Paulsen95) , although this was attributed to higher leucine content in one case(Reference Hamarsland, Laahne and Paulsen95). The differences resulting from intact versus hydrolysed whey protein were much smaller than for intact versus hydrolysed caseins.
At sufficiently high concentration, whey proteins will denature and form a gel when heated. In vitro experiments indicate that whey protein gels are rapidly hydrolysed under gastric conditions, because denaturation makes whey proteins more susceptible to pepsinolysis.(Reference Singh, Oiseth and Lundin96) In conclusion, hydrolysis almost always increases the rate of protein digestion, except where protein digestion rates are already high, as in the case of whey protein.
Whole foods
Heat processing of milk
Heating is a common way to kill pathogens that may be present in raw milk, and heat treatments also extend the shelf life by killing non-pathogenic microorganisms that cause spoilage. In the dairy industry, the most widespread heat treatments for liquid milk are high temperature, short time (HTST) pasteurisation, e.g. 72°C for 15–20 s, and UHT heat treatment, e.g. 140°C for 5 s. The major effect of heat processing milk at >80°C is to denature whey proteins, which adsorb to the surface of casein micelles and weaken gastric coagulation of casein(Reference Vasbinder, Rollema and de Kruif97). Extreme heating can also cause amino acid side chain modifications, as discussed in Section 3.1.
Lacroix et al. (Reference Lacroix, Bon and Bos98) reported post-prandial nitrogen flows in humans after consuming milk treated by heat pasteurisation, UHT treatment or microfiltration (a low-temperature pasteurisation method). Post-prandial plasma amino acids were elevated above baseline levels for all treatments, but the type of milk processing did not produce significant differences. However, a higher rate of deamination and a lower rate of retention was seen with UHT milk, relative to microfiltered milk. The authors suggested that the different nitrogen utilisation pattern for UHT milk could be a result of more rapid gastric emptying and/or heat-related lysine damage(Reference Lacroix, Bon and Bos98). Lysine damage in UHT milk is minor(Reference Rutherfurd and Moughan52), and in vitro simulated digestion studies support the hypothesis of more rapid gastric emptying for UHT milk as a result of weaker gastric coagulation(Reference Ye10).
In another study focused on dairy product processing, Horstman et al. (Reference Horstman, Ganzevles and Kudla72) reported slightly higher post-prandial plasma amino acid levels following consumption of low-fat UHT milk, compared with low-fat pasteurised milk (P=0.066 for the iAUC comparison). In this case, the UHT treatment was applied by direct steam injection, and Horstman et al. (Reference Horstman, Ganzevles and Kudla72) suggested that the observed differences may be even larger with conventional indirect UHT treatment, which exposes milk to high temperatures for a longer time.
Barbé et al. (Reference Barbé, Ménard and Le Gouar65) also examined the effect of a more severe heat treatment (90°C for 10 min) on post-prandial plasma amino acid kinetics in pigs after consumption of liquid milk. Consuming heated milk resulted in slightly higher aminoacidaemia than consuming unheated milk, but the differences were not significant.
Other in vivo and in vitro studies of milk protein digestion have been reviewed by Horstman and Huppertz(Reference Horstman and Huppertz86) and Li et al. (Reference Li, Ye and Singh99). In vitro studies report differences in proteolysis rates between UHT-treated and pasteurised milks(Reference Ye, Liu and Cui74), but in vivo studies have found only small differences in amino acid bioavailability.
Cooking and mincing of beef
Cooking transforms raw meat into an edible form, as well as eliminating pathogens and enhancing flavour. Meat undergoes extensive microstructural and biochemical changes during cooking, including partial or complete denaturation of myofibrillar, sarcoplasmic and connective tissue proteins, shrinkage and expulsion of water, amino acid side chain modifications(Reference Deb-Choudhury, Haines and Harland100) and Maillard reactions between proteins and sugars. These cooking-related changes affect post-prandial nitrogen flows, including plasma amino acid kinetics.
One illustration of cooking effects on post-prandial amino acid kinetics is the study by Bax et al. (Reference Bax, Buffière and Hafnaoui101) using pigs that were fed beef samples cooked for 30 min in a water bath at 60°C, 75°C or 95 °C. Plasma amino acid levels showed significant differences over the first 1.5 h (Figure 7). Kinetic parameters were not significantly different, but it was clear that heating at 75°C led to a faster increase in plasma indispensable amino acids, and heating at 95°C resulted in slower aminoacidaemia.
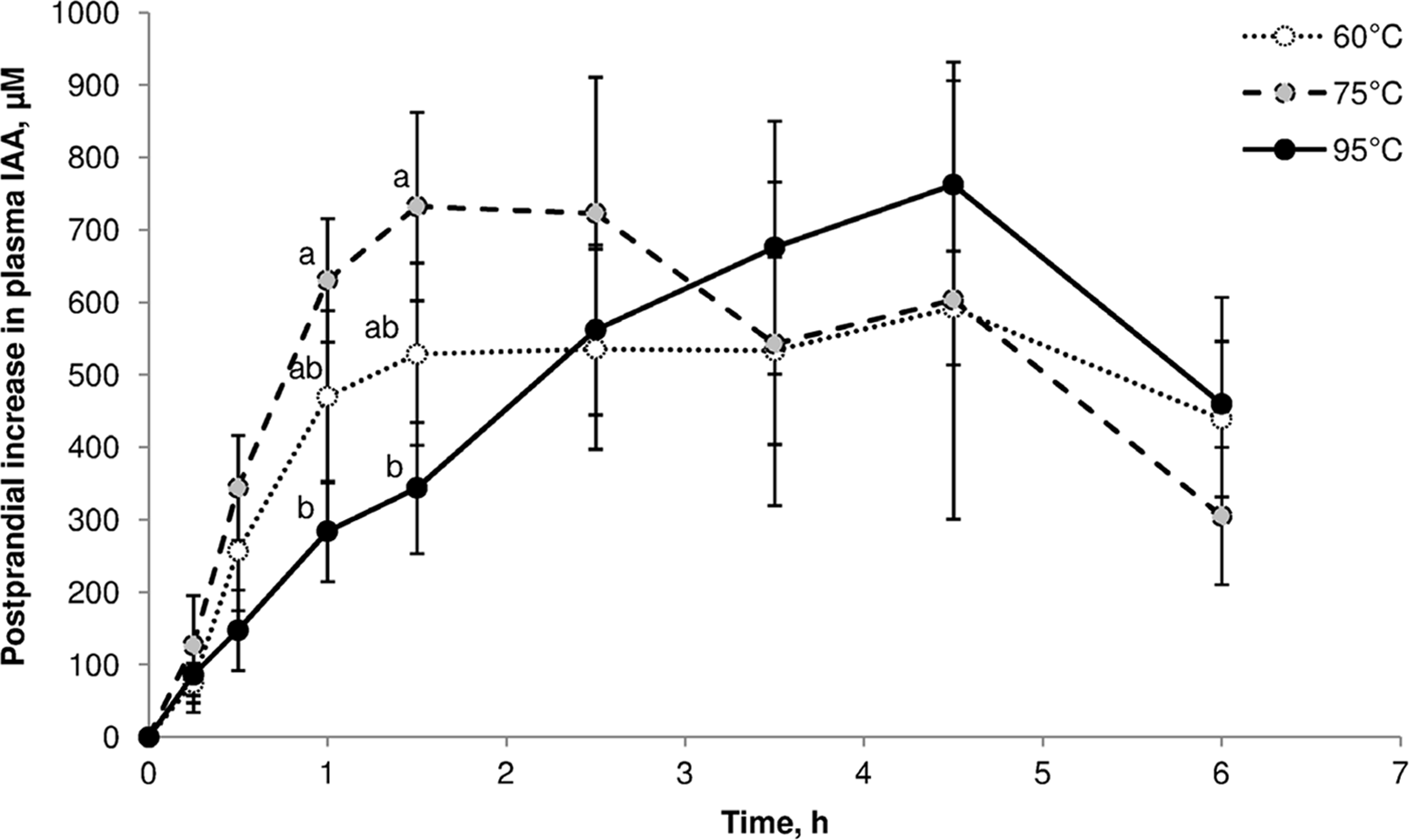
Fig. 7. Post-prandial kinetics of indispensable amino acids in pigs fed beef cooked for 30 min at 60°C, 75°C or 95°C. After Bax et al. (Reference Bax, Buffière and Hafnaoui101), reproduced with permission.
Based on the results of a parallel simulated in vitro digestion study with pork(Reference Bax, Aubry and Ferreira102), Bax et al. (Reference Bax, Buffière and Hafnaoui101) proposed that low-temperature cooking led to protein denaturation, which enhanced proteolysis by exposing more cleavage sites. Cooking at a higher temperature led to protein aggregation, which made cleavage sites less accessible to proteases.
In a comparison of beef cooked at 55°C for 5 min with that cooked at 90°C for 30 min, Oberli et al. (Reference Oberli, Marsset-Baglieri and Airinei103) reported higher ileal nitrogen flow and slightly lower digestibility in healthy young adults for the higher cooking temperature, but plasma amino acid kinetics were not significantly different. The authors hypothesised that the chosen cooking treatments gave rise to meat microstructures that resisted digestion to a similar extent, either through retained native microstructure (cooking at 55°C) or extensive protein aggregation (cooking at 90°C), as suggested by Bax et al. (Reference Bax, Aubry and Ferreira102).
In a subsequent study using the same cooking treatments, Buffière et al. reported significantly different plasma amino acid kinetics for elderly volunteers(Reference Buffière, Gaudichon and Hafnaoui104). Plasma leucine and plasma indispensable amino acids had significantly higher peaks for the beef cooked at 90°C than for beef cooked at 55°C, but the time at which plasma amino acid concentrations peaked was unaffected by cooking method. Whole body protein synthesis was higher with the well-cooked meat(Reference Buffière, Gaudichon and Hafnaoui104). The contrast between the findings of Oberli et al. (Reference Oberli, Marsset-Baglieri and Airinei103) and those of Buffière et al. (Reference Buffière, Gaudichon and Hafnaoui104) highlights the effect of age on digestion, i.e. amino acids from rare and well-cooked meat were equally bioavailable for adults, whereas amino acids in well-cooked meat were more bioavailable for elderly volunteers.
Cooked beef was minced in the work of Buffière et al. (Reference Buffière, Gaudichon and Hafnaoui104), Oberli et al. (Reference Oberli, Marsset-Baglieri and Airinei103) and Bax et al. (Reference Bax, Buffière and Hafnaoui101) to eliminate the effects of inter-individual variation in chewing patterns. Cooking-related differences were attributed to different meat meso- and microstructures. Pennings et al. (Reference Pennings, Groen and van Dijk105) examined the effect of millimetre-scale meat structure, i.e. beef steak versus minced beef, using L-[1-13C]phenylalanine intrinsically labelled beef. Minced beef produced a higher plasma enrichment of L-[1-13C]phenylalanine than steak (Figure 8), suggesting more rapid digestion and absorption. Consequently, whole-body protein balance in the subjects (10 men 74±2 y old) was higher with minced beef.

Fig. 8. Plasma enrichment with L-[1-13C]phenylalanine following consumption of beef steak (open circles) or minced beef (closed circles). After Pennings et al. (Reference Pennings, Groen and van Dijk105), reproduced with permission.
Whereas previous studies compared minced beef cooked at different temperatures and times, or compared minced beef with steak, Prodhan et al. (Reference Prodhan, Pundir and Chiang106) compared beef steaks cooked with different methods: sous vide cooking at 80°C for 6 h or pan frying for 5 min. In young men taking part in the crossover trial, post-prandial plasma amino acid kinetics and hormone levels were not significantly affected by the cooking method. Results from in vitro studies of cooking effects on meat digestion have been reviewed by Bhat et al. (Reference Bhat, Morton and Bekhit107).
As with other food systems, processing of meat influences protein digestion via alterations to microstructure and physical form. Gross alterations such as mincing speed up protein digestion. Mild-to-moderate cooking increases proteolysis rates due to protein denaturation, whereas more extensive cooking can retard proteolysis as a result of extensive protein aggregation
Cooking of egg protein
Egg protein digestion has been studied in vitro (Reference Bhat, Morton and Bekhit108), but few in vivo studies have examined how food processing affects egg protein digestion and absorption. Evenepoel et al. (Reference Evenepoel, Geypens and Luypaerts109) reported that a raw egg meal had substantially lower ileal digestibility than the same meal that had been microwave-cooked (51·3±9·8% versus 90·9±0·8%). Gastric emptying was significantly faster with raw egg, which was attributed to its liquid consistency.
Nau et al. (Reference Nau, Nyemb-Diop and Lechevalier110) took a more detailed look at gastric processing of cooked egg white in pigs, using egg white gels with different microstructures, which were created by adjusting pH and ionic strength before heating(Reference Nyemb, Guérin-Dubiard and Dupont111). Gel structure affected gastric acidification rates and gastric emptying kinetics. The differences were attributed to the effect of egg white gels on the rheological properties of gastric chyme. Parallel in vitro studies suggested that egg white gel structure could affect protein hydrolysis rates(Reference Nyemb, Guérin-Dubiard and Dupont111), as well as the type and amount of peptides released by hydrolysis(Reference Nyemb-Diop, Causeur and Jardin112).
Concluding Remarks
From the in vivo evidence reviewed here, it is clear that several food processing operations affect the kinetics of post-prandial protein digestion and absorption. A wide variety of tissues and metabolic processes are sensitive to amino acid concentrations in peripheral blood, so the impacts of food processing on the body’s response to protein intake are potentially wide ranging.
In many cases, the effect of food processing on digestion and absorption is mediated by protein structure effects at millimetre, micrometre and molecular length scales, which affect the physicochemical behaviour of protein-rich materials in the gastrointestinal tract. The solubility and gastric coagulation potential of food proteins can substantially alter the kinetics of protein digestion and absorption. These co-variate factors are often overlooked when comparing proteins from different sources and may be responsible for some of the variability reported in studies comparing protein sources.
Processing effects on protein digestion rates can be non-linear, as protein denaturation increases susceptibility to proteolysis, and subsequent aggregation inhibits proteolysis. Harsh processing causes chemical reactions that render indispensable amino acids permanently non-bioavailable. Hydrolysis increases the rate of protein digestion and absorption for slowly digestible proteins such as casein, but has little effect for whey proteins, which are rapidly digested and absorbed even when intact.
Food processing effects on protein digestion processes cannot be generalised, because the type and degree of alterations to protein structure depend on specific conditions, especially time, temperature and pH. Few studies have examined the effect of age on protein digestion. The contrasting findings in studies of minced beef digestion by adult(Reference Oberli, Marsset-Baglieri and Airinei103) or elderly(Reference Buffière, Gaudichon and Hafnaoui104) volunteers suggest that processing-related effects on protein digestion may be larger in the elderly.
A lot is known about the digestion of milk and meat products processed in different ways, but the effects of food processing on the digestion of proteins from fish, egg and non-animal sources have received little attention. It is likely that similar principles will apply, i.e. food processes that produce aggregation, hydrolysis or amino acid modifications are likely to alter gastric emptying and/or the accessibility of peptide bonds to proteases. For example, heat-induced aggregation of bean proteins decreases digestibility in rats(Reference Carbonaro, Grant and Cappelloni113). Recent comparisons of soy milk and tofu have found slower in vitro gastric proteolysis with tofu(Reference Reynaud, Lopez and Riaublanc114), corresponding to sustained elevation of intragastric pH in pigs after consuming tofu but not soy milk(Reference Reynaud, Buffière and David115). Additionally, food processes that eliminate the anti-nutritional factors found in pulses are reported to improve overall digestibility(Reference Kashyap, Varkey and Shivakumar116), and may increase the rate of protein digestion and absorption. With increasing public interest in reducing consumption of animal proteins, there is an acute need for better understanding of how food processing affects the digestion and absorption of non-animal proteins from traditional and novel sources.
The connection between protein digestion/absorption kinetics, protein structure and food processing reveals an opportunity to optimise protein digestion rates using carefully designed food processing treatments to improve health outcomes. Research with casein and casein hydrolysates (Table 1) has shown that protein digestion and absorption rates can have large effects on post-prandial insulin secretion profiles. Other effects on splanchnic nitrogen extraction and muscle synthesis are more subtle, and sometimes opposite effects are seen in young versus elderly subjects(Reference Oberli, Marsset-Baglieri and Airinei103,Reference Buffière, Gaudichon and Hafnaoui104,Reference Dangin, Boirie and Guillet117) . Satiety is likely to be influenced by food processes that alter the kinetics of protein digestion and absorption(Reference Barbé, Ménard and Le Gouar66). Food processing to increase satiation could produce foods for appetite control and weight management.
As we age, we need to consume more dietary protein to maintain muscle mass and function, but increasing our protein intake is often a challenge due to declining appetite and the poor palatability of high-protein foods(Reference Baum, Kim and Wolfe118,Reference Dardevet, Mosoni and Savary-Auzeloux119) . There is a need for high-protein foods that are more palatable and less satiating. The protein in these foods should be rapidly digested and absorbed to provide sufficiently high peak plasma amino acid levels to overcome the anabolic resistance or “blunting” of muscle responsiveness that develops with age(Reference Zaromskyte, Prokopidis and Ioannidis120). Such foods will be particularly challenging to produce with plant-derived proteins, which often have lower digestibility and/or lower essential amino acid content(Reference Day, Cakebread and Loveday121).
Food processing technologies are constantly evolving. New technologies such as high hydrostatic pressure processing and microwave-assisted pasteurisation introduce physical or electromagnetic stimuli that denature proteins in different ways to traditional heating or cooking processes(Reference Soni, Samuelsson and Loveday122). These technologies offer new ways to modify protein digestion and absorption rates to beneficially influence physiological responses to food intake. Food processing has strong potential to optimise food protein digestion, but realising this potential will require more human clinical studies in which amino acid absorption rates are measured, and food microstructure is explicitly considered, measured and manipulated.
Acknowledgements
I thank Prof Christiani Jeyakumar Henry for helpful feedback and Dr Ng Heok He for skilful editing, and acknowledge reviewers for constructive and helpful comments.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None
Authorship
S.M.L.: conception, drafting, review and final approval.