Comments and Reply
A response to—“Comment on the evaluation of the constant β relating the contact stiffness to the contact area in nanoindentation for sphero-conical indenters:” Comment to paper “Penetration depth and tip radius dependence on the correction factor in nanoindentation measurements” by J.M. Meza et al. [J. Mater. Res. 23(3), 725 (2008)]
-
- Published online by Cambridge University Press:
- 20 March 2012, pp. 1208-1210
-
- Article
- Export citation
Back Cover (OBC, IBC) and matter
JMR volume 27 issue 11 Cover and Back matter
-
- Published online by Cambridge University Press:
- 15 May 2012, pp. b1-b4
-
- Article
-
- You have access
- Export citation
Articles
Effects of morphological characteristics of Pt nanoparticles supported on poly(acrylic acid)-wrapped multiwalled carbon nanotubes on electrochemical performance of direct methanol fuel cells
-
- Published online by Cambridge University Press:
- 07 June 2012, pp. 2035-2045
-
- Article
- Export citation
Back Cover (OBC, IBC) and matter
JMR volume 27 issue 2 Cover and Back matter
-
- Published online by Cambridge University Press:
- 20 January 2012, pp. b1-b2
-
- Article
-
- You have access
- Export citation
Front Cover (OFC, IFC) and matter
JMR volume 27 issue 19 Cover and Front matter
-
- Published online by Cambridge University Press:
- 20 September 2012, pp. f1-f5
-
- Article
-
- You have access
- Export citation
Articles
An expanding cavity model incorporating pile-up and sink-in effects
-
- Published online by Cambridge University Press:
- 01 December 2011, pp. 132-140
-
- Article
- Export citation
Back Cover (OBC, IBC) and matter
JMR volume 27 issue 18 Cover and Back matter
-
- Published online by Cambridge University Press:
- 04 September 2012, pp. b1-b5
-
- Article
-
- You have access
- Export citation
JMR volume 27 issue 7 Cover and Back matter
-
- Published online by Cambridge University Press:
- 27 March 2012, pp. b1-b4
-
- Article
-
- You have access
- Export citation
Articles
Charge transport in solution-processed zinc tin oxide thin film transistors
-
- Published online by Cambridge University Press:
- 16 May 2012, pp. 2286-2292
-
- Article
- Export citation
Back Cover (OBC, IBC) and matter
JMR volume 27 issue 22 Cover and Back matter
-
- Published online by Cambridge University Press:
- 12 November 2012, pp. b1-b5
-
- Article
-
- You have access
- Export citation
Articles
Nanoscale titania ceramic composite supports for PEM fuel cells
-
- Published online by Cambridge University Press:
- 07 June 2012, pp. 2046-2054
-
- Article
- Export citation
Effect of annealing temperature on the electrical characteristics of Ti–Zn–Sn–O thin-film transistors fabricated via a solution process
-
- Published online by Cambridge University Press:
- 18 May 2012, pp. 2293-2298
-
- Article
- Export citation
Back Cover (OBC, IBC) and matter
JMR volume 27 issue 6 Cover and Back matter
-
- Published online by Cambridge University Press:
- 05 March 2012, pp. b1-b3
-
- Article
-
- You have access
- Export citation
Articles
Fracture modes in micropillar compression of brittle crystals
-
- Published online by Cambridge University Press:
- 13 September 2011, pp. 141-151
-
- Article
- Export citation
Front Cover (OFC, IFC) and matter
JMR volume 27 issue 8 Cover and Front matter
-
- Published online by Cambridge University Press:
- 13 April 2012, pp. f1-f5
-
- Article
-
- You have access
- Export citation
Back Cover (OBC, IBC) and matter
JMR volume 27 issue 19 Cover and Back matter
-
- Published online by Cambridge University Press:
- 20 September 2012, pp. b1-b4
-
- Article
-
- You have access
- Export citation
JMR volume 27 issue 8 Cover and Back matter
-
- Published online by Cambridge University Press:
- 13 April 2012, pp. b1-b4
-
- Article
-
- You have access
- Export citation
Articles
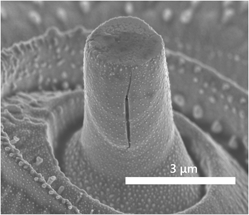
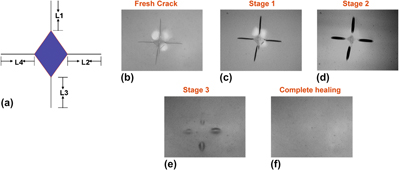
Self-repairable glass seals for solid oxide fuel cells
-
- Published online by Cambridge University Press:
- 14 June 2012, pp. 2055-2061
-
- Article
- Export citation
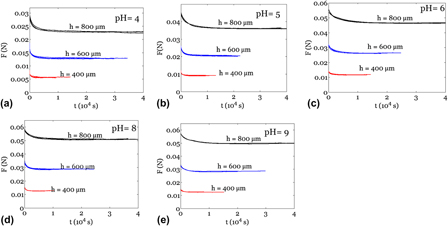
Indentation: A simple, nondestructive method for characterizing the mechanical and transport properties of pH-sensitive hydrogels
-
- Published online by Cambridge University Press:
- 23 November 2011, pp. 152-160
-
- Article
- Export citation
Metallization strategies for In2O3-based amorphous oxide semiconductor materials
-
- Published online by Cambridge University Press:
- 03 July 2012, pp. 2299-2308
-
- Article
- Export citation

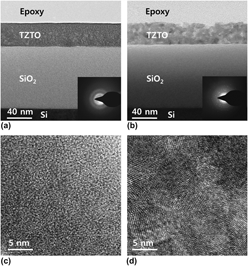
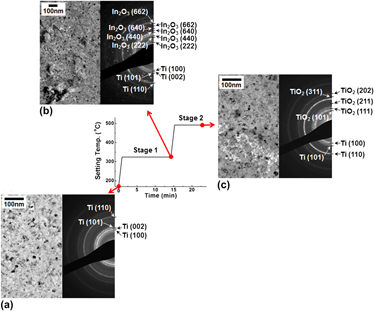
 200 °C), while a-IZO metallized with Mo remains amorphous. The effects of the unstable Ti/IZO interface are shown to include: vacancy injection, enhanced amorphous-to-crystal transformation kinetics, interfacial oxide formation, and the lateral growth on adjacent IZO of rutile TiO
200 °C), while a-IZO metallized with Mo remains amorphous. The effects of the unstable Ti/IZO interface are shown to include: vacancy injection, enhanced amorphous-to-crystal transformation kinetics, interfacial oxide formation, and the lateral growth on adjacent IZO of rutile TiO