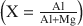The adsorption of organic molecules on mineral matrices might have played a fundamental role in processes that led to the emergence of life. We investigated the adsorption properties of the nucleobases adenine, cytosine, uracil and hypoxanthine on magnesium oxide (MgO), determining the single solute batch equilibrium adsorption isotherms. Langmuir-type isotherms were fitted to data, assuming a rapid reversible equilibration of adsorption, demonstrated effectively through desorption experiments. The Langmuir equilibrium adsorption constant K and the amount of the solute per unit of adsorbent mass necessary to complete the monolayer b were calculated. The results indicate that MgO is a good adsorbent for nucleobases (adenine > uracil > hypoxantine > cytosine), suggesting a role of metal oxides in concentrating biomolecules in prebiotic conditions that might have favoured the passage from geochemistry to biochemistry.