Diegogattaite (IMA2012-096), Na2CaCu2Si8O20·H2O, is a new mineral from the Wessels mine in the Kalahari manganese fields of South Africa. It occurs as a minor phase with other copper-bearing silicates, Cu-rich pectolite, sugilite, quartz, aegirine and undifferentiated Fe-Mn oxides. Diegogattaite is pale turquoise through teal blue. It is found as sub-mm sized grains in a main crystalline patch 3–4 mm in size, and is currently known from only one sample. The mineral is transparent with a vitreous lustre and may have a good cleavage on {001}. It is brittle, with an uneven fracture and a very pale-blue streak. It is non-fluorescent in short- and long-wave UV light and has an estimated Mohs hardness of ∼5–6. Diegogattaite is biaxial (–), α = 1.598(2), β = 1.627(2), γ = 1.632(2); 2Vmeas = 44.0(6)°, 2Vcalc = 44.5°; dispersion: strong r < v, orientation: X = b, Y ≈ ⊥(001), Z ≈ a; pleochroism X colourless << Y ≈ Z blue green. The calculated density is 3.10 g/cm3. Electron-microprobe analysis gave: Na2O 8.07, CaO 7.3, CuO 20.5, FeO 0.36, SiO262.4, H2O(calc) 2.34, total 100.97 wt.%. A charge-balanced formula on the basis of 21 oxygen a.p.f.u. is: Na2.00Ca1.00Cu1.98Fe0.04Si7.99H2O21. Diegogattaite is monoclinic, space group C2/m, a = 12.2439(6) Å, b = 15.7514(4) Å, c = 10.6008(3) Å, β = 125.623(2)°, V = 1661.87(10) Å3 and Z = 4. The five strongest lines in the X-ray powder pattern are [dobs in Å (Iobs)(hkl)]: 4.25(75)(002,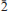 22,220), 3.951(77)(040), 3.261(100)(
22,220), 3.951(77)(040), 3.261(100)(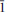 31,
31,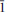 13), 2.898(89)(042,
13), 2.898(89)(042,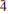 03,003), 2.332(66)(331,
03,003), 2.332(66)(331,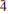 43,
43,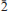 62,260,043). The crystal structure of diegogattaite was determined by single-crystal X-ray diffraction to final agreement indices of R1 = 0.027, wR2 = 0.071 and GoF = 1.090. It represents a completely new silicate topology based upon a double-sheet of SiO4 tetrahedra composed of connected 6482 cages. The structure of diegogattaite is related to those of synthetic nanoporous Na-Cu-Si-O-(OH)-H2O (CuSH) compounds, which are of interest to the solid-state chemistry community as potential ion-exchangers, catalysts and molecular sieves. The structure of diegogattaite forms a bridge between these structures and those of the gillespite-group minerals, including wesselsite. The close spatial association of wesselsite and diegogattaite suggests a possible reaction between them that may point to a synthetic route for the production of novel alkaline-earth-based nanoporous copper silicates.
62,260,043). The crystal structure of diegogattaite was determined by single-crystal X-ray diffraction to final agreement indices of R1 = 0.027, wR2 = 0.071 and GoF = 1.090. It represents a completely new silicate topology based upon a double-sheet of SiO4 tetrahedra composed of connected 6482 cages. The structure of diegogattaite is related to those of synthetic nanoporous Na-Cu-Si-O-(OH)-H2O (CuSH) compounds, which are of interest to the solid-state chemistry community as potential ion-exchangers, catalysts and molecular sieves. The structure of diegogattaite forms a bridge between these structures and those of the gillespite-group minerals, including wesselsite. The close spatial association of wesselsite and diegogattaite suggests a possible reaction between them that may point to a synthetic route for the production of novel alkaline-earth-based nanoporous copper silicates.

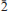 22,220), 3.951(77)(040), 3.261(100)(
22,220), 3.951(77)(040), 3.261(100)(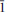 31,
31,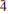 03,003), 2.332(66)(331,
03,003), 2.332(66)(331,