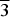Crystal structures of A0.50SbFe(PO4)3(A=Mn, Cd) phases, obtained by solid state reaction at 920 °C, were determined at room temperature from X-ray powder diffraction (XRD) using the Rietveld method. The structures of the two samples are of the Nasicon-type with the R3 space group. Hexagonal cell parameters for A=Mn and Cd are: a=8.375(1) Å, c=21.597(2) Å and a=8.313(1) Å, c=21.996(2) Å, respectively. From XRD data, it is difficult to unambiguously distinguish between Cd2+ and Sb5+ ions in Cd0.50SbFe(PO4)3 and between Mn2+ and Fe3+ cations in Mn0.50SbFe(PO4)3. Nevertheless the overall set of cation–anion distances within the Nasicon framework clearly shows that the cation distribution can be illustrated by the {[A0.50]3a[◻0.50]3b}M1SbFe(PO4)3 (A=Mn, Cd) crystallographic formula. The divalent A2+ cations and vacancies are ordered within the two positions, 3a and 3b, of the M1 sites. Structure refinements show also a quasi-ordered distribution of Sb5+ and Fe3+ ions within the Nasicon framework. Thus, each A(3a)O6(A=Mn, Cd) octahedron shares two faces with two Fe3+O6 octahedra and each vacancy (◻(3b)O6) site is located between two Sb5+O6 octahedra.