Adsorption of Mo(VI) on 2-0.2-μm size fraction of sodium-saturated kaolinite at 25 ± 2°C and at a constant pH of 7.00 ± 0.05 was studied. The kaolinite sample was pretreated to remove any surface oxide and hydroxide coatings. The initial concentrations of Mo in solution ranged from 1 to 11 mg/liter in a NaClO4 background electrolyte at a constant ionic strength of 0.09 ± 0.01. Calculations of speciation using the GEOCHEM computer program indicated that under experimental conditions Mo(VI) was mainly in the MoO42− form. The experimental conditions were also shown to fulfill the requirements for applying the Langmuir equation in interpreting adsorption data. The Langmuir parameter for the adsorption maximum, n°, and the affinity parameter, 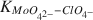 were computed to be 3.33 × 10−4 mole/ mole of adsorbent and 5.969 × 105, respectively. The large affinity parameter indicated that the Na-saturated kaolinite surface has a very high affinity for MoO42− ions relative to ClO4− ions.
were computed to be 3.33 × 10−4 mole/ mole of adsorbent and 5.969 × 105, respectively. The large affinity parameter indicated that the Na-saturated kaolinite surface has a very high affinity for MoO42− ions relative to ClO4− ions.