Published online by Cambridge University Press: 02 April 2024
Adsorption of Mo(VI) on 2-0.2-μm size fraction of sodium-saturated kaolinite at 25 ± 2°C and at a constant pH of 7.00 ± 0.05 was studied. The kaolinite sample was pretreated to remove any surface oxide and hydroxide coatings. The initial concentrations of Mo in solution ranged from 1 to 11 mg/liter in a NaClO4 background electrolyte at a constant ionic strength of 0.09 ± 0.01. Calculations of speciation using the GEOCHEM computer program indicated that under experimental conditions Mo(VI) was mainly in the MoO42− form. The experimental conditions were also shown to fulfill the requirements for applying the Langmuir equation in interpreting adsorption data. The Langmuir parameter for the adsorption maximum, n°, and the affinity parameter, KMoO42−−ClO4−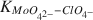 were computed to be 3.33 × 10−4 mole/ mole of adsorbent and 5.969 × 105, respectively. The large affinity parameter indicated that the Na-saturated kaolinite surface has a very high affinity for MoO42− ions relative to ClO4− ions.
were computed to be 3.33 × 10−4 mole/ mole of adsorbent and 5.969 × 105, respectively. The large affinity parameter indicated that the Na-saturated kaolinite surface has a very high affinity for MoO42− ions relative to ClO4− ions.
Исследовалась адсорбция Mo(VI) на фракции размером 2-0,2 μм каолинита, насыщен-ного натрием при температуре 25 ± 2°C и при постоянной величине рН равной 7,00 ± 0,05. Образец каолинита обработывался так, чтобы удалить все окисные и гидроокисные поверх-ностные покрытия. Начаьные концентрации Мо в растворе распределялись от 1 до 11 мг/литр в электролите NaClO4 при постоянной ионовой силе равной 0,09 ± 0,01. Вычисления количества новых вилов при помощи програмы ГЭОХЕМ указывали на то, что в этих экспериментальных условиях Mo(VI) находился в основном в форме MoO22−. Показано, что экспериментальные условия выполняли необходимые условия для использования уравнения Лангмюра для интерпретации данных адсорбции. Параметр Лангмюра для максимума адсорбции, n0, и параметр подобия,  , были вычислены как 3,33 × 10−4 моля/моль адсорбента и 5,969 × 105, соответственно. Значительный параметр подобия указывал на то, что поверхность каолинита, насыщенного Na, имеет очень высокое подобие для ионов MoO42− по отношению к ионом ClO4−. [E.G.]
, были вычислены как 3,33 × 10−4 моля/моль адсорбента и 5,969 × 105, соответственно. Значительный параметр подобия указывал на то, что поверхность каолинита, насыщенного Na, имеет очень высокое подобие для ионов MoO42− по отношению к ионом ClO4−. [E.G.]
Es wurde die Adsorption von Mo(VI) an die 2-0,2 μm Fraktion von Na-gesättigtem Kaolinit bei 25 ± 2°C und einem konstanten pH von 7,00 ± 0.05 untersucht. Die Kaolinitprobe wurde vorbehandelt, um oxidische und hydroxidische Oberflächenbeläge zu entfernen. Die ursprünglichen Konzentrationen an Mo in der Lösung reichten von 1–11 mg/Liter in einer NaClO4 Elektrolytlösung mit einer konstanten Ionenstärke von 0,09 + 0,01. Die Berechnungen mittels GEOCHEM-Programm deuten darauf hin, daß unter den experimentellen Bedingungen Mo(VI) hauptsächlich als MoO42- vorlag. Es zeigte sich auch, daß die experimentellen Bedingungen so waren, daß sie die Anforderungen für die Anwendung der Langmuir-Gleichung bei der Interpretation der Adsorptionsdaten erfüllt haben. Der Langmuir-Parameter für das Adsorptionsmaximum, n°, und der Affinitätsparameter, 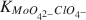 , wurden, auf 3,33 × 10-4 Mole/Mole für den Adsorbenten bzw. mit 5,969 × 105 berechnet. Der große Affinitätsparameter deutete darauf hin, daß die Na-gesättigte Kaolinitoberfläche eine große Affinität für MoO42--Ionen im Vergleich zu ClO4--Ionen hat. [U.W.]
, wurden, auf 3,33 × 10-4 Mole/Mole für den Adsorbenten bzw. mit 5,969 × 105 berechnet. Der große Affinitätsparameter deutete darauf hin, daß die Na-gesättigte Kaolinitoberfläche eine große Affinität für MoO42--Ionen im Vergleich zu ClO4--Ionen hat. [U.W.]
On a étudié l'adsorption de Mo(VI) sur une fraction de kaolinite saturée de sodium de taille 2-0,2 μm à 25 ± 2°C at à un pH constant de 7,00 ± 0.05. L’échantillon de kaolinite avait été traité à l'avance pour enlever toutes couches oxides et hydroxides. Les concentrations de Mo initiales dans la solution s’étendaient d'l à 11 mg/litre dans un électrolyte d'arrière plan de NaClO4 à une force ionique constante de 0,09 ± 0,01. Des calculs de spéciation employant le programme GEOCHEM ont indiqué que sous les conditions expérimentales Mo(VI) était principalement dans la forme MoO42-. On a aussi montré que les conditions expérimentales ont satisfait les exigences pour appliquer l’équation de Langmuir dans l'interprétation des données d'adsorption. Le paramètre de Langmuir pour le maximum d'adsorption, n°, et le paramètre d'affinité,  , ont été computés être 3,33 × 10−4 mole/mole d'adsorbant et 5,969 × 105, respectivement. Le grand paramètre d'affinité a indiqué que la surface de kaolinite saturée de Na a une affinité très élevée pour les ions MoO42− relativement aux ions ClO4− [D.J.]
, ont été computés être 3,33 × 10−4 mole/mole d'adsorbant et 5,969 × 105, respectivement. Le grand paramètre d'affinité a indiqué que la surface de kaolinite saturée de Na a une affinité très élevée pour les ions MoO42− relativement aux ions ClO4− [D.J.]