Feroxyhyte (δ′-FeOOH) is a relatively uncommon Fe oxide mineral and one of the few phases in the system Fe2O3-H2O for which thermodynamic properties are not known. In natural occurrences, it is always fine-grained, although samples with larger particle sizes and better crystallinity (labeled as δ-FeOOH) can be prepared in the laboratory. This contribution presents a thermochemical study on a series of feroxyhyte samples. One is fine-grained and poorly crystalline, similar to natural materials, while the other three are of better crystallinity. The enthalpy of formation of feroxyhyte at 298.15 K is −547.4±1.3kJ mol−1 for the poorly crystalline sample (surface area 88 m2/g), and −550.6±1.4, −550.9±1.3, and −552.6±1.2 kJ mol−1 for the samples with better crystallinity. The entropy of feroxyhyte can be estimated only crudely, because it is influenced to a great extent by its magnetic properties, particle size, and structural disorder. The $S_{298}^{\rm{o}}$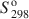 of feroxyhyte is estimated here to be 65±5 J K−1 mol−1. The Gibbs free energy of the reaction feroxyhyte → hematite + liquid water is −7.4 to −12.6 kJ mol−1 at 298.15 K. The Gibbs free energy of formation (${\rm{\Delta }}G_{\rm{f}}^{\rm{o}}$
of feroxyhyte is estimated here to be 65±5 J K−1 mol−1. The Gibbs free energy of the reaction feroxyhyte → hematite + liquid water is −7.4 to −12.6 kJ mol−1 at 298.15 K. The Gibbs free energy of formation (${\rm{\Delta }}G_{\rm{f}}^{\rm{o}}$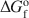 ) of the fine-grained, poorly crystalline feroxyhyte is −478.1±2.0 kJ mol−1 at 298.15 K. Since this sample is closest in its physical properties to natural feroxyhyte, this ${\rm{\Delta }}G_{\rm{f}}^{\rm{o}}$
) of the fine-grained, poorly crystalline feroxyhyte is −478.1±2.0 kJ mol−1 at 298.15 K. Since this sample is closest in its physical properties to natural feroxyhyte, this ${\rm{\Delta }}G_{\rm{f}}^{\rm{o}}$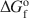 value should be used in thermodynamic modeling related to processes involving naturally occurring feroxyhyte. In terms of Gibbs free energy and enthalpy, feroxyhyte is very similar to lepidocrocite and maghemite, and, like these two phases, has no thermodynamic stability field in the system Fe2O3-H2O, except possibly at the nanoscale.
value should be used in thermodynamic modeling related to processes involving naturally occurring feroxyhyte. In terms of Gibbs free energy and enthalpy, feroxyhyte is very similar to lepidocrocite and maghemite, and, like these two phases, has no thermodynamic stability field in the system Fe2O3-H2O, except possibly at the nanoscale.