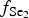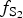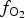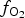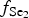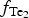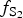The Gaching high-sulfidation epithermal deposit in the Maletoyvayam ore field features a wide range of Se-containing minerals and selenides, as well as complex gold oxides, Au tellurides (calaverite, krennerite) and native gold typical for epithermal deposits. Pyrite included in quartzites and quartz-alunite rocks was probably formed during an early stage of the ore-forming process. During the following Au-rich stage, the 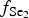 $f_{{\rm S}{\rm e}_{\rm 2}}$/
$f_{{\rm S}{\rm e}_{\rm 2}}$/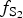 $f_{{\rm S}_{\rm 2}}$ increased with
$f_{{\rm S}_{\rm 2}}$ increased with 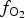 $f_{{\rm O}_{\rm 2}}$ being relatively high, resulting in the formation of very rare compounds that have not been previously described in nature. These include Au2Te4(Se,S)3, Se3Te2, AuSe and Au(Te,Se,S) phases. The Au2Te4(Se,S)3 compounds have some variations in composition: the complete isomorphic series between Au2Te4Se3 and Au2Te4S3 was observed. The gold and Au-minerals at the main ore stage can be stable within a range of log
$f_{{\rm O}_{\rm 2}}$ being relatively high, resulting in the formation of very rare compounds that have not been previously described in nature. These include Au2Te4(Se,S)3, Se3Te2, AuSe and Au(Te,Se,S) phases. The Au2Te4(Se,S)3 compounds have some variations in composition: the complete isomorphic series between Au2Te4Se3 and Au2Te4S3 was observed. The gold and Au-minerals at the main ore stage can be stable within a range of log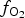 $f_{{\rm O}_{\rm 2}}$ of −27.3 and atmospheric oxygen (?); log
$f_{{\rm O}_{\rm 2}}$ of −27.3 and atmospheric oxygen (?); log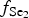 $f_{{\rm S}{\rm e}_{\rm 2}}$ between −12.4 and −5.7; log
$f_{{\rm S}{\rm e}_{\rm 2}}$ between −12.4 and −5.7; log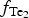 $f_{{\rm T}{\rm e}_{\rm 2}}$ between −10.5 and −7.8; and log
$f_{{\rm T}{\rm e}_{\rm 2}}$ between −10.5 and −7.8; and log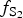 $f_{{\rm S}_{\rm 2}}$ between −12.8 and −6.8 (at 250°C). The increasing oxygen fugacity during the final stage of mineralization resulted in the formation of complex Sb,As,Te,S-bearing Au oxides. Gold-oxide formation occurs due to oxidation of Au-tellurides. The final products of this process are newly-formed secondary mustard gold and Te–Se solid solutions.
$f_{{\rm S}_{\rm 2}}$ between −12.8 and −6.8 (at 250°C). The increasing oxygen fugacity during the final stage of mineralization resulted in the formation of complex Sb,As,Te,S-bearing Au oxides. Gold-oxide formation occurs due to oxidation of Au-tellurides. The final products of this process are newly-formed secondary mustard gold and Te–Se solid solutions.