Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Vorobyev, Sergey
Likhatski, Maxim
Romanchenko, Alexander
Maksimov, Nikolai
Zharkov, Sergey
Krylov, Alexander
and
Mikhlin, Yuri
2018.
Colloidal and Deposited Products of the Interaction of Tetrachloroauric Acid with Hydrogen Selenide and Hydrogen Sulfide in Aqueous Solutions.
Minerals,
Vol. 8,
Issue. 11,
p.
492.
Palyanova, G. A.
Tolstykh, N. D.
Zinina, V. Yu.
Kokh, K. A.
Seryotkin, Yu. V.
and
Bortnikov, N. S.
2019.
Synthetic Gold Chalcogenides in the Au–Te–Se–S System and Their Natural Analogs.
Doklady Earth Sciences,
Vol. 487,
Issue. 2,
p.
929.
Zhao, Jing
and
Pring, Allan
2019.
Mineral Transformations in Gold–(Silver) Tellurides in the Presence of Fluids: Nature and Experiment.
Minerals,
Vol. 9,
Issue. 3,
p.
167.
Shapovalova, M
Tolstykh, N
and
Bobrova, O
2019.
Chemical composition and varieties of sulfosalts from gold mineralization in the Gaching ore occurrence (Maletoyvayam ore field).
IOP Conference Series: Earth and Environmental Science,
Vol. 319,
Issue. 1,
p.
012019.
Tolstykh, Nadezhda D.
Palyanova, Galina A.
Bobrova, Ol’ga V.
and
Sidorov, Evgeny G.
2019.
Mustard Gold of the Gaching Ore Deposit (Maletoyvayam Ore Field, Kamchatka, Russia).
Minerals,
Vol. 9,
Issue. 8,
p.
489.
Kalinin, Arkadii A.
Savchenko, Yevgeny E.
and
Selivanova, Ekaterina A.
2019.
Mustard Gold in the Oleninskoe Gold Deposit, Kolmozero–Voronya Greenstone Belt, Kola Peninsula, Russia.
Minerals,
Vol. 9,
Issue. 12,
p.
786.
Sidorov, Evgeny G.
Borovikov, Andrey A.
Tolstykh, Nadezhda D.
Bukhanova, Daria S.
Palyanova, Galina A.
and
Chubarov, Valery M.
2020.
Gold Mineralization at the Maletoyvayam Deposit (Koryak Highland, Russia) and Physicochemical Conditions of Its Formation.
Minerals,
Vol. 10,
Issue. 12,
p.
1093.
Palyanova, G. A.
2020.
Gold and Silver Minerals in Sulfide Ore.
Geology of Ore Deposits,
Vol. 62,
Issue. 5,
p.
383.
Tolstykh, Nadhezda D.
Tuhý, Marek
Vymazalová, Anna
Plášil, Jakub
Laufek, František
Kasatkin, Anatoly V.
Nestola, Fabrizio
and
Bobrova, Olga V.
2020.
Maletoyvayamite, Au3Se4Te6, a new mineral from Maletoyvayam deposit, Kamchatka peninsula, Russia.
Mineralogical Magazine,
Vol. 84,
Issue. 1,
p.
117.
Palyanova, Galina
Mikhlin, Yuri
Zinina, Veronika
Kokh, Konstantin
Seryotkin, Yurii
and
Zhuravkova, Tatyana
2020.
New gold chalcogenides in the Au–Te–Se–S system.
Journal of Physics and Chemistry of Solids,
Vol. 138,
Issue. ,
p.
109276.
Anisimova, Galina S.
Kondratieva, Larisa A.
and
Kardashevskaia, Veronika N.
2020.
Characteristics of Supergene Gold of Karst Cavities of the Khokhoy Gold Ore Field (Aldan Shield, East Russia).
Minerals,
Vol. 10,
Issue. 2,
p.
139.
Tolstykh, Nadezhda
Bukhanova, Daria
Shapovalova, Maria
Borovikov, Andrey
and
Podlipsky, Maksim
2021.
The Gold Mineralization of the Baranyevskoe Au-Ag Epithermal Deposit in Central Kamchatka.
Minerals,
Vol. 11,
Issue. 11,
p.
1225.
Gurin, G. V.
2021.
Geophysical prospecting for epithermal gold deposits (a case study from the Maletoivayam gold ore field, Kamchatka Peninsula).
LITHOSPHERE (Russia),
Vol. 21,
Issue. 1,
p.
116.
Sejkora, Jiří
Dolníček, Zdeněk
Zachariáš, Jiří
Ulmanová, Jana
Šrein, Vladimír
and
Škácha, Pavel
2022.
Mineralogical and Fluid Inclusion Evidence for Reworking of Au Mineralization by Ag-Sb-Base Metal-Rich Fluids from the Bytíz Deposit, Příbram Uranium and Base-Metal Ore District, Czech Republic.
Minerals,
Vol. 12,
Issue. 12,
p.
1539.
Berdnikov, Nikolai
Kepezhinskas, Pavel
Konovalova, Natalia
and
Kepezhinskas, Nikita
2022.
Formation of Gold Alloys during Crustal Differentiation of Convergent Zone Magmas: Constraints from an AU-Rich Websterite in the Stanovoy Suture Zone (Russian Far East).
Geosciences,
Vol. 12,
Issue. 3,
p.
126.
Tolstykh, N. D.
Bortnikov, N. S.
Shapovalova, M. O.
and
Shaparenko, E. O.
2022.
The Role of Hydrocarbons in the Formation of Epithermal Gold–Silver Deposits in Kamchatka, Russia.
Doklady Earth Sciences,
Vol. 507,
Issue. 2,
p.
994.
Kalinin, Yu. A.
Borovikov, A. A.
Maacha, L.
Zouhair, M.
Palyanova, G. A.
and
Zhitova, L. M.
2022.
Au–Pd Mineralization and Ore-Forming Fluids of the Bleïda Far West Deposit (Anti-Atlas, Morocco).
Geology of Ore Deposits,
Vol. 64,
Issue. S2,
p.
S237.
Tolstykh, Nadhezda D.
Tuhý, Marek
Vymazalová, Anna
Laufek, František
Plášil, Jakub
and
Košek, Filip
2022.
Gachingite, Au(Te1–xSex) 0.2 ≈ x ≤ 0.5, a new mineral from Maletoyvayam deposit, Kamchatka peninsula, Russia.
Mineralogical Magazine,
Vol. 86,
Issue. 2,
p.
205.
Palyanova, Galina
Beliaeva, Tatiana
Kokh, Konstantin
Seryotkin, Yurii
Moroz, Tatyana
and
Tolstykh, Nadezda
2022.
Characterization of synthetic and natural gold chalcogenides by electron microprobe analysis, X‐ray powder diffraction, and Raman spectroscopic methods.
Journal of Raman Spectroscopy,
Vol. 53,
Issue. 5,
p.
1012.
Kalinin, Yu. A.
Borovikov, A. A.
Maacha, L.
Zuhair, M.
Palyanova, G. A.
and
Zhitova, L. M.
2022.
Au-Pd mineralization and ore-forming fluids of the Bleïda Far West deposit (Anti-Atlas, Morocco).
LITHOSPHERE (Russia),
Vol. 22,
Issue. 5,
p.
644.
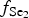 $f_{{\rm S}{\rm e}_{\rm 2}}$/
$f_{{\rm S}{\rm e}_{\rm 2}}$/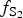 $f_{{\rm S}_{\rm 2}}$ increased with
$f_{{\rm S}_{\rm 2}}$ increased with 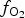 $f_{{\rm O}_{\rm 2}}$ being relatively high, resulting in the formation of very rare compounds that have not been previously described in nature. These include Au2Te4(Se,S)3, Se3Te2, AuSe and Au(Te,Se,S) phases. The Au2Te4(Se,S)3 compounds have some variations in composition: the complete isomorphic series between Au2Te4Se3 and Au2Te4S3 was observed. The gold and Au-minerals at the main ore stage can be stable within a range of log
$f_{{\rm O}_{\rm 2}}$ being relatively high, resulting in the formation of very rare compounds that have not been previously described in nature. These include Au2Te4(Se,S)3, Se3Te2, AuSe and Au(Te,Se,S) phases. The Au2Te4(Se,S)3 compounds have some variations in composition: the complete isomorphic series between Au2Te4Se3 and Au2Te4S3 was observed. The gold and Au-minerals at the main ore stage can be stable within a range of log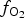 $f_{{\rm O}_{\rm 2}}$ of −27.3 and atmospheric oxygen (?); log
$f_{{\rm O}_{\rm 2}}$ of −27.3 and atmospheric oxygen (?); log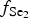 $f_{{\rm S}{\rm e}_{\rm 2}}$ between −12.4 and −5.7; log
$f_{{\rm S}{\rm e}_{\rm 2}}$ between −12.4 and −5.7; log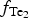 $f_{{\rm T}{\rm e}_{\rm 2}}$ between −10.5 and −7.8; and log
$f_{{\rm T}{\rm e}_{\rm 2}}$ between −10.5 and −7.8; and log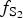 $f_{{\rm S}_{\rm 2}}$ between −12.8 and −6.8 (at 250°C). The increasing oxygen fugacity during the final stage of mineralization resulted in the formation of complex Sb,As,Te,S-bearing Au oxides. Gold-oxide formation occurs due to oxidation of Au-tellurides. The final products of this process are newly-formed secondary mustard gold and Te–Se solid solutions.
$f_{{\rm S}_{\rm 2}}$ between −12.8 and −6.8 (at 250°C). The increasing oxygen fugacity during the final stage of mineralization resulted in the formation of complex Sb,As,Te,S-bearing Au oxides. Gold-oxide formation occurs due to oxidation of Au-tellurides. The final products of this process are newly-formed secondary mustard gold and Te–Se solid solutions.