INTRODUCTION
Japanese encephalitis (JE), a mosquito-borne arboviral infection caused by flavivirus, is prevalent in South-East Asia and the Western Pacific regions. It was estimated that about 50 000 cases of JE and more than 10 000 deaths in Asia were reported annually between 1973 and 1990 [Reference Igarashi1, 2]. JE not only causes death but also leads to permanent and psychiatric sequale. The case-fatality rate varies across regions, ranging from 10% to 30% [Reference Tsai3, Reference Solomon4].
JE virus can be found in many kinds of vertebrates and is transmitted by an infected mosquito that has previously sucked blood from an infected animal. Pigs are the most important amplifiers and reservoirs. With the advent of vaccination and pesticide control, the morbidity in some countries such as Japan, Korea, and Taiwan has been reduced over the past 30 years. For example, since mass vaccination was implemented in Taiwan in 1968, the incidence of JE has declined from 2·05/10 000 in 1967 to 0·03/10 000 in 1997 [Reference Wu5]. Similar findings were observed in Japan, Korea and China. Despite this, JE is still one of the major vector-borne and emerging viral diseases, particularly in the Southern Asia and Western Pacific regions [Reference Mackenzie6], posing a threat to humans, partly because mass vaccination administered to humans was incomplete and partly because epidemiological patterns and the distribution of JE have changed after the implementation of mass vaccination.
From the socio-ecological viewpoint, an outbreak of JE may be facilitated by two factors, i.e. global climate change and the modulation of agriculture (such as adoption of paddy cultivation, use of pesticides and the creation of modern pig farms). Temperature and precipitation have been reported to be associated with the density of mosquitoes [Reference Gingrich7, Reference Lin and Lu8] and are also highly related to occurrence of JE according to the Konno transmission model [Reference Konno9], which suggests that the cyclic outbreak of JE among pigs and humans is demonstrated by collecting serum specimens from pigs at weekly interval and isolating the JE virus from mosquitoes (Culex tritaeniorhynchus) during the summer of 1964. Such a relationship is also supported by regular seasonal epidemics in northern South-East Asia, China, and Korea. Based on the above finding, both temperature and precipitation may be adopted as proxy variables for representing the level of mosquito density. However, there is a paucity of direct empirical evidence showing the temporal relationship between climate variability and occurrence of JE.
Like Korea and Japan, Taiwan initiated universal vaccination for children after 1968 and, since then, the incidence rate of JE has decreased greatly. However, the chance of a JE epidemic occurring in Taiwan may still be higher than in both other countries due to different coverage rates of JE immunization for pigs. In Korea and Japan, pigs are immunized with killed vaccine or live attenuated vaccine. There is no compelling and comprehensive immunization programme for pigs in Taiwan. In most farms, the vaccination is only applied to sows for the first breeding (because the fetuses from infected sows are often stillborn or mummified).
Due to the severe consequences of JE, surveillance for early detection of epidemics based on climate factors in combination with other factors may be of paramount importance in a country with a high vaccination rate for children but without a comprehensive immunization programme for pigs. The aims of this study were therefore:
(1) to report the secular trend of reported cases and confirmed cases of JE and also of climate variability during the period from 1991 to 2005 in Taiwan;
(2) to investigate whether time trend and seasonal pattern of JE has changed over the past 15 years;
(3) to elucidate the temporal relationship between the lagged climate variables (precipitation and temperature) and the occurrence of JE taking into account seasonal pattern, time trend, vaccination rate, pig density, area variation and time dependence of time-series number of JE cases.
METHODS
Definition of reported and confirmed JE cases
A JE case was routinely reported according to two definitions: (1) presentation with clinical symptoms related to encephalomeningitis, combined with fever, malaise, nausea, convulsion, headache, nuchal rigidity or flaccid paralysis; or (2) asymptomatic but highly suspected by a physician, or conforming to epidemiological evidence. A JE case was further confirmed by laboratory diagnosis with haemagglutination inhibition and neutralization tests.
Data sources
Several archival data were collected to perform the following time-series analysis, including:
(1) JE cases: reported and confirmed cases during 1991–2005 were collected from the routinely reported infectious disease system [10]. A total of 4058 reported cases were identified between 1991 and 2005. Of 4058 reported cases, a total of 287 confirmed cases were identified following the above definition.
(2) Climate variability: information on monthly temperature and precipitation was obtained from the meteorological stations scattered throughout Taiwan island [11]. Data on monthly basis were accrued between 1991 and 2005.
(3) The pig density was estimated as number of pigs per km2, which was derived from the Annual Statistical Report of Agriculture [12].
(4) Population data was abstracted from the annual statistic report issued by the Ministry of the Interior [13]. As population density varies by area, population size in each area between 1991 and 2005 was also collected from the Ministry of the Interior in order to provide person-years for the following time-series generalized autoregressive Poisson regression analysis.
(5) Data on vaccination status was derived from the Ministry of Health's annual health statistical report [14].
Statistical analysis
To assess the impact of the lagged climate variable on occurrence of confirmed JE with adjustment for seasonal pattern and time trend, pig density, vaccination rate and geographic area, we used a generalized autoregressive Poisson regression model which assumed that the occurrence of JE is rare and followed Poisson distribution.
This method has been applied to model a time-series of the monthly number of cases of poliomyelitis in the United States [Reference Zeger15] and is also documented in chapter 6 of the textbook by Fahrmeir & Tutz [Reference Fahrmeir and Tutz16]. Another merit of using a generalized autoregressive Poisson regression model is to consider time dependence of JE cases in successive time by incorporation of autoregressive order, which has been defined as ‘the regression of JE counts at time t on the JE counts at earlier time t – 1 (i.e y tvs. y t – l)’. The model form is written as follows:

where y t=number of JE confirmed cases by month at time t; x 1, …, x p=covariates such as pig density, variation rate and geographical area; t=linear trend; m 1, …, m 11=seasonal pattern (modelled as 11 dummy variables); y t – j=autoregressive order j (number of JE confirmed cases at time t – j) and ln (PY)=person-years (offset).
represents the lagged temperature at time t – k and the lagged precipitation at time t – i multiply by two regression coefficients, v k and πi, respectively; β1, …, βp, δ1, ω1, …, ω12 and γj represent coefficients for the corresponding covariates in the above equation.
To check where there is extra-Poisson variation (over-dispersion), heterogeneity factor (deviance divided by degrees of freedom) was used as an indicator to assess whether it is larger than 1.
Regarding the details of the other covariates considered in the multivariable regression model mentioned above, seasonal months were incorporated as 11 dummy variables (m 1−m 11) by taking December as the baseline group. Only linear time trend was considered because the quadratic term was not statistically significant. It is well know that paddy cultivation is another crucial factor for occurrence of JE. However, we did not collect information on this variable. As the distribution of paddy cultivation varies with geographic area, we used a proxy variable, 23 geographic areas of Taiwan, to capture the heterogeneity of JE occurrence caused by this factor. As location of pig farm also varies with area, the incorporation of pig density from 23 geographic areas may also capture the heterogeneity of location of pig farm. We also considered assessing whether the effect of climate factors on JE may vary with 23 areas since climate factors vary across geographic areas. However, lack of statistical power may result if 23 areas are stratified. We therefore grouped 23 areas into four main areas and checked whether the results based on the four main areas are identical to those based on 23 areas. The statistically significant level was set at 0·05.
RESULTS
Secular trend and seasonal pattern of JE
The upper curve in Figure 1 (top panel) shows the secular trend of reported cases from January 1991 to December 2005. Apparently, there was a remarkable seasonal pattern, clustering between May and October. The seasonal pattern between 1991 and 1997 remained stable. However, the variability of seasonal pattern after 1998 tended to be larger than before. The lower curve in Figure 1 (top panel) is the corresponding time trend for confirmed cases. A similar curved shape, although the rate was smaller, was observed for confirmed cases. The three smaller panels in Figure 1 represent the monthly confirmed cases aggregated from each 5-year period. The confirmed JE cases in 1991–1995 clustered between June and October whereas those between 1996 and 2000 and those between 2001 and 2005 clustered from May to August.
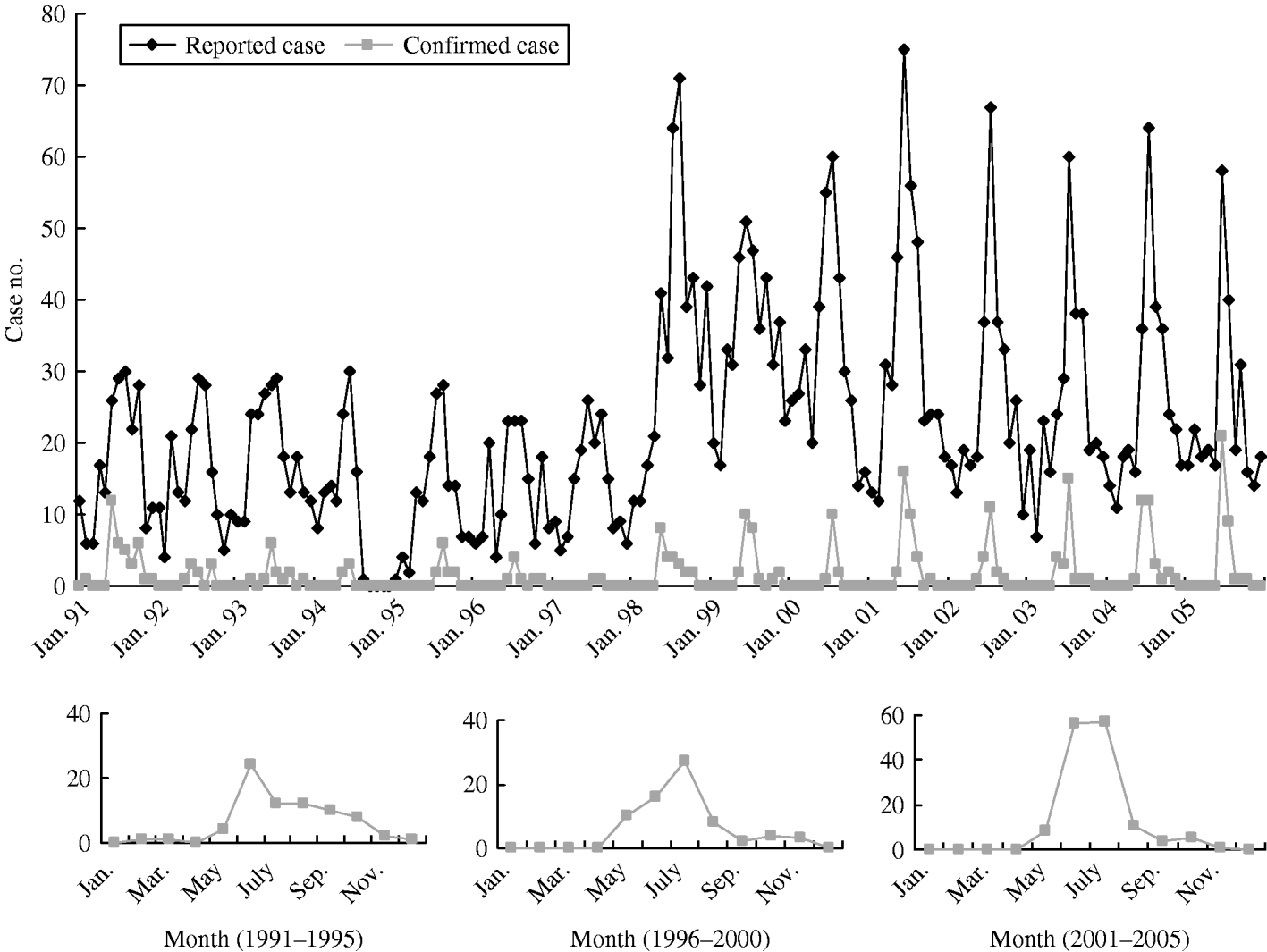
Fig. 1. Secular trend of reported cases and confirmed cases of Japanese encephalitis by month between 1991 and 2005. The three smaller panels represent the monthly confirmed cases aggregated from each 5-year period.
Figure 2 also shows the secular trends regarding temperature and precipitation. The fluctuation of both patterns shows remarkable seasonal type as observed for JE cases.
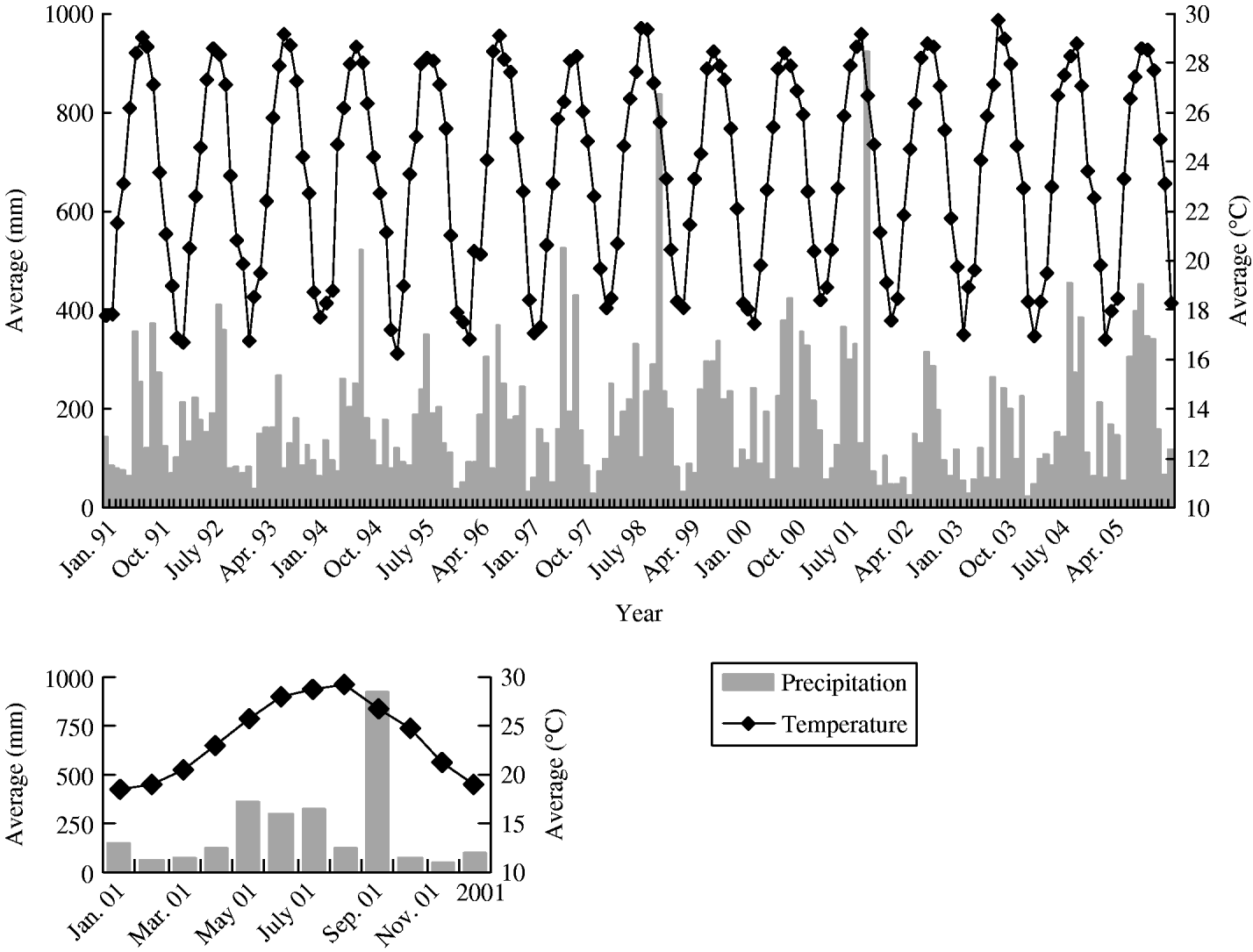
Fig. 2. Secular trend of precipitation and temperature by month between 1991 and 2005.
Analysis of generalized autoregressive Poisson regression model
Univariate analysis for the associations between confirmed cases and relevant factors after adjustment for geographic areas are shown in the upper panel of Table 1 by generalized autoregressive Poisson regression model. The lagged temperature and precipitation variables at t – 2 (time lag 2) were found to be statistically significantly associated with the occurrence of JE at time t. The pig density was statistically positively associated with number of confirmed cases. For seasonal factors, occurrence of JE shows a remarkable increase from May to August. The relationship between vaccination rate and counts of confirmed cases was not statistically significant.
Table 1. Results of univariate analysis and multivariate analysis of the lagged climate variable in association with occurrence of Japanese encephalitis

n.a., Not available for estimation because of the sparse number of cases.
* Adjusted for geographic area, autoregressive order t – 1, seasonal pattern and time trend.
† Adjusted for geographic area, autoregressive order t – 1.
Table 1 also presents the multivariate analysis adjusting for variables in each other, including pig density, vaccination rate, area, autoregressive order, seasonal factors and linear time trend. The joint effect of the lagged t – 2 of temperature and the lagged t – 1 of precipitation on occurrence of cases at time t was statistically significant. After adjustment for temperature and precipitation, the influence of pig density on confirmed cases still remained. It should be noted that as the autoregressive second order was not significant only the first order was retained in the univariate and multivariate analysis.
For extra-Poisson variation, the heterogeneity factor, calculated by using deviance divided by degrees of freedom, was equal to 0·4. This suggests a lack of heterogeneity (P=1·00).
In order to assess whether the effect of the lagged temperature and precipitation on JE may vary by different geographic characteristics, 21 areas (excluding two areas without JE-confirmed cases during 1991–2005) were grouped into four areas (Fig. 3) (Northern area: Taipei county, Ilan county, Taoyuan county, Hsinchu county, Taipei city and Hsinchu city. Middle area: Miaoli county, Taichung county, Changhua county, Nantou county, Yunlin county, Chiai county, Taichung city, and Chiai city. Southern area: Tainan county, Kaohsiung county, Pingtung county, Kaohsiung city, and Tainan city. Eastern area: Taitung county and Hualien county). The likelihood ratio test was performed to verify whether the model with four merged areas is different from that consisting of 21 areas. However, there was a lack of significant difference between the two models. The results of univariate analysis using four areas are identical to that using 21 areas.

Fig. 3. Map of Taiwan (grouped into four areas).
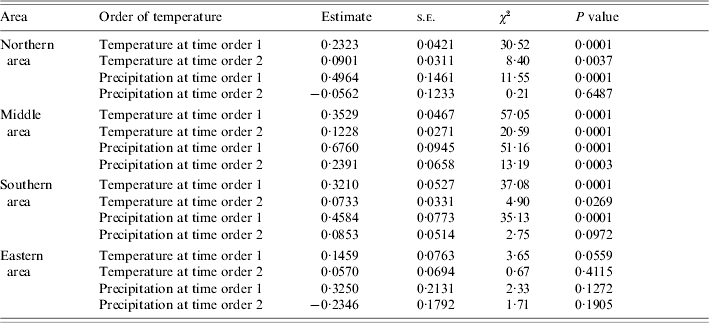
Table 2 shows the effect of the lagged temperature and precipitation variables on JE by the four areas obtained from the generalized autoregressive Poisson regression model. The corresponding effect of temperature in the Northern, Middle and Southern areas was found at time lag 2. The effect of precipitation on JE in the Northern and Southern areas was observed at time lag 1. The corresponding effect of precipitation in the Middle area was found at time lag 2. However, the impact of both factors on JE was not substantial in the Eastern area.
Table 2. Estimated results of univariate analysis for the effect of time order of temperature and precipitation
DISCUSSION
There are two main findings in the current study. First, seasonal pattern related to occurrence of JE cases between 1991 and 2005 clusters between May and August and may last longer, up to October in Taiwan (smaller panels in Fig. 1). The earlier shift to May since 1991 is similar to the phenomenon found in the previous study that the seasonal pattern in JE occurrence tended to shift from August in the 1960s to June in 1980s [Reference Wu5]. This change may also reflect the higher likelihood of being infected with JE through a mosquito bite, as individual mosquito density may also have undergone ecological change parallel to temperature and precipitation as shown in the current study. The current universal vaccination for JE in children has been conducted between the March and May annually. Based on the above findings on the change of occurrence of first JE cases, advancing the time for routine vaccination may be considered in order to prevent JE cases in humans.
Second, the effects of time-lagged temperature and precipitation on the occurrence of JE were quantified in this study with adjustment for other factors including seasonal pattern, time trend, pig density, 23 geographic areas representing location of farm and paddy cultivation, and vaccination coverage rate for humans. However, it may be argued that mosquito density has not been controlled in the current study. As mentioned before, as our study focuses on the effect of ecological components such as climate factors on the occurrence of JE cases we assumed temperature and precipitation as two proxy variables for mosquito density. The change of mosquito density may be in parallel to the ecological change of temperature and precipitation.
Although the previous study has indicated the relationship of climate such as precipitation and temperature to JE, the exact temporal sequence with adjustment for relevant factors has never been addressed. The significant effect of the lagged climate variables on JE occurrence in humans has been demonstrated in the present study using a generalized autoregressive model taking seasonal factors, time trend, pig density, vaccination rate and area into account. This result suggests that JE occurrence in the present month is related to temperature and precipitation of 1–2 months previously which is analogous to the findings of Bi et al. [Reference Bi17], although, several correlates were not controlled in their study. Our finding was also consistent with the Konno transmission model of JE [Reference Konno9] and supports the hypothesis that temperature may be regarded as catalyst in forming viraemia of pigs and in facilitating multiplication of mosquitoes, and precipitation may be regarded as the promoter for increasing the number of mosquitoes.
The results were also consistent with the previous findings. An epidemiological survey in Sri Lanka demonstrated an increase in mosquitoes following heavy rainfall [Reference Peiris18, Reference Peiris19]. This further led to the presence of seroconversion and JE in humans. Another study in Thailand indicated that non-immune sentinel pigs in the dry hot season were not susceptible to infection until several weeks after the first rains of the weather season [Reference Gingrich20]. One study in China also found that reduced rainfall led to a decrease in the C. tritaeniorhynchus mosquito population in 1980 and 1981. This further resulted in a decrease in JE in Beijing in 1982 and 1983 [Reference Wang21].
It should be noted that the above temporal relationship may vary by different geographic areas and other factors, such as pig density and vaccine coverage rate. The finding on time lag 1 of climate variables for the Southern area in Taiwan suggests that it takes 1 month for temperature and precipitation to effect occurrence of JE in humans. High density of pig breeders and the mosquito population in these areas may account for shorter duration because both factors may facilitate reaching threshold density of the mosquito population.
There is one concern as to whether an increased incidence in JE cases since 1998 has been attributed to changing the diagnostic criteria of JE. A surveillance system of JE commenced in 1990 and clinical diagnostic criteria have not been changed since then; therefore we could hardly attribute such an increase to the changed diagnostic criteria since 1998. However, we do speculate whether surveillance awareness due to foot-and-mouth disease (FMD) is one of the major contributions. In 1997, FMD swept through swine husbandry in Taiwan resulting in large-scale slaughtering of pigs and huge financial losses for the pork industry. This may account for a sharp increase in JE cases since 1998. Other ecological viewpoints may also explain the change in level after 1998. Global climate change is an interesting topic on climatic factors in association with health. For instance, the El Niño Southern Oscillation (ENSO) is a classical example. The relation between El Niño and intense rainfall is strong in several areas during 1997–1998. In Taiwan, the average temperature has increased since 1998 and the precipitation was also elevated in 1998, 2001 and 2005. Both may account for the change in level after 1998.
Vaccination is effective in reducing occurrence of JE but this does not mean the vaccination rate can effect the occurrence of JE cases after vaccine introduction. A universal vaccination schedule for JE has been built up over the past 30 years in Taiwan. The vaccination has been regularly conducted in children and the overall coverage is up to 80% in each county since 1968 in Taiwan. There is a lack of significant difference of vaccination across time and counties. This might be why vaccination rate was not associated with JE occurrence in Table 1.
In fact, the change of two climate factors may reflect the evolution of mosquito density. The implication for JE surveillance is that if one can add the change of temperature and precipitation into established information on seasonal factors and pig density, one can perceive in advance whether a JE epidemic will break out. The incorporation of two proxy variables for mosquito density is particularly useful for monitoring high-incidence areas such as the Southern area.
In conclusion, the seasonal pattern on occurrence of JE cases clusters between May and August during the period from 1991 to 2005 in Taiwan. The earlier shift to May suggests the current schedule of vaccination may need to be advanced earlier. In each geographic area, monitoring temperature and precipitation, two possible proxy variables for mosquito density, in conjunction with seasonal factors and pig density is of assistance in forecasting JE epidemics.
DECLARATION OF INTEREST
None.







