The emphasis on breakfast eating for overall health can be traced back to the 16th century in Europe(Reference Arndt Anderson1). Described as the most important meal of the day, breakfast provides nutrients for the body after overnight fasting(Reference Gibney, Barr and Bellisle2). Recently, several studies demonstrate that breakfast skippers may have a higher risk of developing diabetes(Reference Mekary, Giovannucci and Willett3), hypertension(Reference Lee, Kim and Hwang4), CVD(Reference Cahill, Chiuve and Mekary5,Reference Ridker, Glynn and Hennekens6) and cancer(Reference Yokoyama, Onishi and Hosoda7), relative to breakfast eaters. Of note, all of the aforementioned chronic conditions or diseases are associated with chronic inflammation(Reference Ridker, Glynn and Hennekens6,Reference Kaptoge and Angelantonio8–Reference Wang, Bao and Liu10) . Two recent cross-sectional studies reported that consumption of breakfast foods was associated with lower inflammation, assessed via serum C-reactive protein (CRP)(Reference di Giuseppe, Di Castelnuovo and Melegari11) or glycoprotein acetyls(Reference Guinter, Campbell and Patel12). However, these studies were limited by an indirect assessment of breakfast consumption(Reference di Giuseppe, Di Castelnuovo and Melegari11) or a small sample size (n 644)(Reference Guinter, Campbell and Patel12). The study by di Giuseppe et al. (Reference di Giuseppe, Di Castelnuovo and Melegari11) assessed participants’ breakfast consumption by determining whether they reported consumption of foods which were considered ‘typical Italian breakfast foods’ (e.g. milk, coffee, crisp bread and breakfast cereals). This assessment method is limited by the uncertainty on whether those ‘typical Italian breakfast foods’ were also eaten at lunch or dinner, other than breakfast.
Thus, we conducted a cross-sectional study to examine whether breakfast consumption frequency was associated with inflammatory status among 70 092 Chinese adults, after adjustment for overall dietary quality, sleep parameters, medical history, blood lipid profile and other potential confounders. Chronic inflammation, measured via plasma or serum CRP, is an accepted risk factor for many common conditions, including CVD(Reference Cushman, Arnold and Psaty13), type 2 diabetes(Reference Pradhan, Manson and Rifai14) and hypertension(Reference Sesso, Buring and Rifai15). The link between CRP and CVD is well established, while risk estimates vary slightly, even after adjusting for age, ethnicity, sex and other CVD risk factors. Since 2003, the Centers for Disease Control and Prevention and the American Heart Association (AHA) have recommended that CRP concentrations in the blood be quantified ‘as an adjunct’ to the traditional CVD risk factors (e.g. total cholesterol, HDL-cholesterol and systolic and diastolic blood pressure)(Reference Pearson, Mensah and Alexander16). However, even in the absence of hyperlipidaemia, CRP has proven to be an effective indicator of CVD risk(Reference Ridker, Glynn and Hennekens6).
Upon existing evidences as aforementioned, regular breakfast consumption has been associated with reduced risk of chronic diseases, such as CVD. To understand the potential mechanism underlying this relationship, we aim to examine whether breakfast frequency was associated with inflammatory status, as assessed by high-sensitivity CRP concentration, among individuals who were free of CVD and cancer.
Methods
Study population
The current study was based on two large cohorts: the Kailuan I study and the Kailuan II study, which have been described previously(Reference Wu, Jin and Li17,Reference Li, Cheng and Cui18) . In brief, Kailuan I is a population-based prospective cohort launched in Tangshan City, China, in 2006, with 101 510 Chinese adults aged 18 years or older who lived in the Kailuan community. Kailuan II was launched in 2008 and includes 35 856 participants living in the same community, who were not enrolled in the Kailuan I study. All participants of the Kailuan studies have undergone routine assessments, including physical examination, anthropometry and laboratory measures, at baseline. Biennial follow-ups were conducted to update information on lifestyle and health status. Information on habitual dietary intakes was assessed with a semi-quantitative FFQ in 2014. Among 74 529 participants who finished the FFQ, 911 were excluded due to missing CRP values, 2279 were excluded due to history of CVD or cancer and 1247 were excluded with clinical CRP concentrations >10 mg/l at the study visit (Fig. 1). Thus, the current study was based on 70 092 participants (57 631 men and 12 458 women; average age 52 ± 14 years).
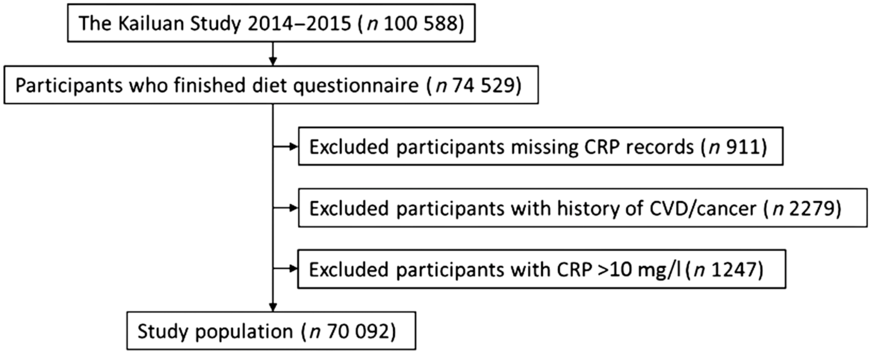
Fig. 1 Flow chart of participant inclusion. CRP, C-reactive protein
Assessment of breakfast frequency
Breakfast frequency was assessed using the question ‘how many days do you usually consume breakfast in a typical week?’. The possible answers were ‘no breakfast’, ‘1–2 times weekly’, ‘3–5 times weekly’ and ‘breakfast every day’. Participants were then categorised into four groups based on their responses.
Assessment of C-reactive protein concentration
Fasting (8–12 h) venous blood samples were drawn from the participants by nurses and transfused into vacuum tubes containing EDTA during the study visit. Following collection, all blood samples were analysed the same day in the Central Laboratory of Kailuan General Hospital. Plasma CRP concentration was measured using a high-sensitivity, particle-enhanced immunonephelometry assay (Cias Latex CRP-H; Kanto Chemical Co. Inc.) with a lower limit of detection of 0·1 mg/l. The intra-assay CV was 6·53 %, and the inter-assay CV was 4·78 %. Further details regarding the assessment of plasma CRP can be found elsewhere(Reference Wu, Huang and Jin19).
We used two cut-off points for high CRP concentration based on its clinical relevance to CVD risk, as recommended by Centers for Disease Control and Prevention and AHA(Reference Pearson, Mensah and Alexander16): ≥1·0 mg/l (moderate-to-high CVD risk group) and ≥3 mg/l (high CVD risk group).
Assessment of covariates
Dietary data were collected with a validated semi-quantitative FFQ in 2014(Reference Wu, An and Li20,Reference Li, Song and Pan21) . The semi-quantitative FFQ includes thirty-three food items and seven condiments and asks how often the participants consumed each food item over the preceding year, with the options of never, daily, weekly or monthly. The consumption amount of each food was also assessed via the FFQ in the unit of liangs (50 g/liang). Diet quality was assessed by adherence to AHA recommendation (referred to as ‘AHA diet score’ in the current study), which was calculated based on the following five components: the consumption of fruits and vegetables, fish, Na, sweets, sugar-sweetened beverages and whole grains, as described previously. The score ranges from 0 (worst) to 5 (best)(Reference Wu, An and Li20).
During the study visit, weight and height were measured, and BMI was calculated as weight (kg)/height (m2). Systolic blood pressure was measured twice after participants were seated statically and averaged for further analysis(Reference Wu, Jin and Li17). An autoanalyser (Hitachi 747; Hitachi) was used to measure blood glucose, HDL-cholesterol and LDL-cholesterol, as detailed previously(Reference Wu, An and Li20,Reference Ma, Gurol and Huang22) .
Questionnaires were used to collect information on age, sex, lifestyle (smoking status, alcohol consumption and physical activity), medication use history (the use of antihypertensive drugs and/or antidiabetic drugs) and sociodemographic data (education level, marital status and occupation type)(Reference Wu, An and Li20). Diabetic status was determined by fasting blood glucose > 7·0 mmol/l or the use of antidiabetic drug. Smoking status was divided into three categories: never, former and current. Alcohol consumption was divided into four categories: never, former, current ≤1 drink/d and current >1 drink/d. Physical activity level was assessed with the validated International Physical Activity Questionnaire, short version, and participants were categorised as inactive, moderately active or vigorously active(Reference Macfarlane, Lee and Ho23). Insomnia status was assessed using a Chinese version of the Athens Insomnia Scale(Reference Chung, Kan and Yeung24,Reference Li, Huang and Hou25) . Questions included self-reported usual total hours of actual sleep at night and self-reported snoring + self-reported breathing stops(Reference Li, Huang and Hou25).
Statistical analysis
Data were analysed with SAS version 9.4 (SAS Institute, Inc.). All statistical tests were two-sided. We used generalised linear models to calculate adjusted means and 95 % CI for CRP concentration. Because the distribution of CRP concentration was highly skewed, log-transformed CRP was used in the statistical analysis as the outcome variable in three models. In the models, we log-transformed CRP to better normalise the distribution. We then transformed back CRP measurements to exponential form to show clinically meaningful values to provide a clearer interpretation in the result section. Model 1 adjusted for age, sex and total energy intake. Model 2 further adjusted for diet score, BMI, education level, occupation type, marital status, smoking status, alcohol use, sleep duration, insomnia, snoring and physical activity. Model 3 adjusted for the covariates in model 2 with the addition of the use of antihypertensive and antidiabetic drugs, systolic blood pressure, fasting blood glucose, LDL-cholesterol and HDL-cholesterol. A secondary sensitivity analysis was performed in addition to the three models to exclude participants with diabetes. Least squares means and corresponding CI were then transformed back to the standard CRP units. Trend test was performed by treating breakfast frequency as a continuous variable in the regression model. We tested multiplicative interactions between breakfast pattern and age, sex, BMI and the AHA diet score, in relation to CRP concentration, after adjusting for covariates in model 3.
To further assess the association between breakfast frequency and inflammatory status, we categorised participants according to their serum CRP concentration. Two cut-off points were used. Participants with CRP ≥ 1·0 mg/l were identified as at moderate risk of developing CVD and those with CRP ≥ 3·0 mg/l were at high risk of developing CVD(Reference Pearson, Mensah and Alexander16). Logistic regression was used to calculate OR and 95 % CI for increased inflammation (CRP) across different breakfast frequency groups.
Results
Among the 70 092 participants in the current study, 86 % reported breakfast consumption every day and 8 % reported no breakfast (Table 1).
Table 1 Baseline characteristics by breakfast consumption frequency

SBP, systolic blood pressure; CRP, C-reactive protein; FBG, fasting blood glucose.
* Genomic mean.
Compared with the ‘breakfast everyday’ group, individuals who skipped breakfaster at least 1 d (referred to as ‘breakfast skippers’ in the current study) were more likely to be men and younger in age and had higher education levels and total energy intake (Table 1).
CRP concentration was significantly higher among individuals in the ‘no breakfast’ group, relative to those who consumed breakfast every day (adjusted mean 1·33 v. 1·07 mg/l; P-trend < 0·001), after adjusting for age, sex, total energy intake, diet quality score, BMI, education level, occupation type, marital status, smoking status, alcohol use, physical activity, sleep parameters, blood pressure, fasting glucose concentration, antihypertensive drug use, antidiabetic drug use and lipid profiles (Table 2). Further, not eating breakfast increased the odds of elevated CRP. The adjusted OR, for the ‘no breakfast’ group v. the ‘breakfast everyday’ group, were 1·86 (95 % CI 1·73, 2·00) for CRP ≥ 1·0 mg/l and 1·27 (95 % CI 1·15, 1·40) for CRP ≥ 3·0 mg/l (P-trend < 0·001 for both; Table 3).
Table 2 Adjusted means and 95 % CI of C-reactive protein concentration, by breakfast consumption frequency

Model 1 adjusted for age (years), sex and total energy intake (kJ/d).
Model 2 adjusted for age (years), sex, total energy intake (kJ/d), American Heart Association diet score, BMI (kg/m2), education level (elementary school, high school or college or above), occupation type (coal miner, other blue-collar jobs or other), marital status (single or married), smoking status (never, former or current), alcohol use (never, former, current <1 drink/d and current >1 drinks/d), sleep duration (<6, 6–7, 7–8 or ≥8 h/d), insomnia (yes/no), snoring (never/rare, occasional or frequent) and physical activity (inactive, moderately active or vigorously active).
Model 3 adjusted for age, sex, total energy intake (kJ/d), American Heart Association diet score, BMI, education level (elementary school, high school or college or above), occupation type (coal miner, other blue-collar jobs or other), marital status, smoking status (never, former or current), alcohol use (never, former, current <1 drink/d and current >1 drinks/d), antihypertensive drug, antidiabetic drug, sleep duration (<6, 6–7, 7–8 or ≥8 h/d), insomnia (yes/no), snoring (never/rare, occasional or frequent) and physical activity (inactive, moderately active or vigorously active), systolic blood pressure (mmHg), fasting blood glucose status (‘normal’ (< 100 mmol/l), ‘impaired fasting glucose’ (100–125 mmol/l) or ‘Diabetes’ (>126 mmol/l or use of hypoglycaemic treatment)), LDL-cholesterol and HDL-cholesterol (mmol/l).
P values for difference from ‘breakfast everyday’ group are indicated as * <0·05, ** <0·01,*** <0·001.
Table 3 OR and 95 % CI for high C-reactive protein (CRP), across breakfast frequency groups

Model 1 adjusted for age (years), sex and total energy intake (kJ/d).
Model 2 adjusted for age(years), sex, total energy intake (kJ/d), diet score, BMI (kg/m2), education level (elementary school, high school or college or above), occupation type (coal miner, other blue-collar jobs or other), marital status (single or married), smoking status (never, former or current), alcohol use (never, former, current <1 drink/d and current >1 drinks/d), sleep duration (<6, 6–7, 7–8 or ≥8 h/d), insomnia (yes/no), snoring (never/rare, occasional or frequent) and physical activity (inactive, moderately active or vigorously active).
Model 3 adjusted for age (years), sex, total energy intake (kJ/d), diet score, BMI (kg/m2), education level (elementary school, high school or college or above), occupation type (coal miner, other blue-collar jobs or other), marital status, smoking status (never, former or current), alcohol use (never, former, current <1 drink/d and current >1 drinks/d), antihypertensive drug, antidiabetic drug, sleep duration (<6, 6–7, 7–8 or ≥8 h/d), insomnia (yes/no), snoring (never/rare, occasional or frequent), physical activity (inactive, moderately active or vigorously active), systolic blood pressure (mmHg), fasting blood glucose status (‘normal’ (< 100 mmol/l), ‘impaired fasting glucose’ (100–125 mmol/l) or ‘Diabetes’ (>126 mmol/l or use of hypoglycaemic treatment)), LDL-cholesterol and HDL-cholesterol (mmol/l).
Age and diet quality significantly modified the associations between breakfast frequency and CRP (P for interaction < 0·001 for both; Table 4). The associations were more pronounced among older adults, relative to younger adults (P for interaction < 0·001). Interestingly, significant association between breakfast skipping and elevated CRP concentration was only observed in those with poor diet quality score, rather than those with a healthy diet quality (P for interaction < 0·001).
Table 4 Adjusted means and 95 % CI of C-reactive protein concentration, by breakfast frequency, stratified by age, sex and diet quality score

Adjusted for age (years), sex, total energy intake (kJ/d), American Heart Association diet score, BMI (kg/m2), education level (elementary school, high school or college or above), occupation type (coal miner, other blue-collar jobs or other), marital status, smoking status (never, former or current), alcohol use (never, former, current <1 drink/d and current >1 drinks/d), antihypertensive drug, antidiabetic drug, sleep duration (<6, 6–7, 7–8 or ≥8 h/d), insomnia (yes/no), snoring (never/rare, occasional or frequent), physical activity (inactive, moderately active or vigorously active), systolic blood pressure (mmHg), fasting blood glucose status (‘normal’ (<100 mmol/l), ‘impaired fasting glucose’ (100–125 mmol/l) or ‘Diabetes’ (>126 mmol/l or use of hypoglycaemic treatment)), LDL-cholesterol and HDL-cholesterol (mmol/l).
* P value for difference from ‘breakfast everyday’ group is indicated as <0·05.
With the exclusion of participants with diabetes, adjusted means of CRP concentration were still significantly higher in ‘no breakfast’ group as compared with ‘breakfast everyday’ groups (1·26 v. 1 mg/l; P < 0·001) (see online supplementary material, Supplemental Table).
Discussion
In this large-scale community-based study, we found that individuals who consumed no breakfast had a higher plasma CRP concentration, compared with those who consumed breakfast every day. This association was independent of demographic, anthropometric, socioeconomic and dietary variables. Elevated inflammation, measured via CRP(Reference Sproston and Ashworth26), is consistently associated with increased relative risk of CVD(Reference Cushman, Arnold and Psaty13,Reference Pai, Pischon and Ma27) and has more recently been associated with depressive symptoms(Reference Valkanova, Ebmeier and Allan28) and an increased risk of cancer(Reference Allin and Nordestgaard9) and all-cause mortality(Reference Li, Zhong and Cheng29). Habitually skipping breakfast has also been associated with higher odds of having chronic inflammation.
Recently, one cross-sectional study including 644 participants in the Cancer Prevention Study-3 Diet Assessment Sub-study assessed breakfast consumption and inflammatory status using glycoprotein acetyl as the inflammatory indicator(Reference Guinter, Campbell and Patel12). Individuals who ate breakfast for 5 d had higher glycoprotein acetyl (β 0·21; 95 % CI 0·03, 0·40) when compared with those who consumed breakfast on all 6 d. However, no association was found for other exposure groups, which could be due to the small sample size of those groups(Reference Guinter, Campbell and Patel12). Interestingly, irregularity in breakfast consumption, as assessed by the intra-individual sd of time at first intake of the day, was significant associated with high glycoprotein acetyl(Reference Guinter, Campbell and Patel12). Consistently, in a previous cross-sectional study including 18 777 Italian adults, a breakfast score was developed based on the consumption of ‘typical Italian breakfast foods’, including milk, coffee, tea, yogurt, crispbread/rusks, breakfast cereals, brioche, biscuits, honey, sugar and jam(Reference di Giuseppe, Di Castelnuovo and Melegari11). Relative to individuals in the lowest breakfast foods score quintile, those in the highest quintile had lower CRP (1·35 v. 1·57 mg/l) and lower odds of having CRP ≥ 1·0 mg/l (adjusted OR 0·83, 95 % CI 0·73, 0·93)(Reference di Giuseppe, Di Castelnuovo and Melegari11).
Our findings are consistent with previous studies that link breakfast behaviour and chronic disease risk. Habitually skipping breakfast has been associated with increased risk of CVD(Reference Cahill, Chiuve and Mekary5,Reference di Giuseppe, Di Castelnuovo and Melegari11,Reference Kubota, Iso and Sawada30) , type II diabetes(Reference Mekary, Giovannucci and Willett3,Reference Uemura, Yatsuya and Hilawe31) , obesity(Reference Horikawa, Kodama and Yachi32) and cancer(Reference Yokoyama, Onishi and Hosoda7). For example, in a study based on the National Health and Nutrition Examination Survey III, participants who did not consume breakfast had a significantly increased risk of CVD mortality, after adjustment for age, sex, race, socioeconomic status, dietary and lifestyle factors, BMI and other conventional cardiovascular risk factors(Reference Rong, Snetselaar and Xu33). Similarly, in the Health Professionals Follow-Up Study, including 26 902 men, Cahill et al. (Reference Cahill, Chiuve and Mekary5) found that habitual breakfast skippers had a 27 % higher risk of myocardial infarction as compared with breakfast eaters during 16 years of follow-up. A meta-analysis also reported that skipping breakfast is associated with obesity (pooled OR 1·75, 95 % CI 1·57, 1·95)(Reference Horikawa, Kodama and Yachi32). While the mechanisms connecting breakfast consumption and chronic disease risk are not fully understood, increased inflammation in breakfast skippers has been suggested as one potential pathway connecting the two(Reference Nas, Mirza and Hägele34).
CVD and the aforementioned chronic conditions are associated with elevated CRP(Reference Ridker, Glynn and Hennekens6,Reference Kaptoge and Angelantonio8) . Hence, our findings that habitual breakfast skipping is associated with chronic inflammation (increased plasma CRP) support the hypothesis that breakfast skipping may increase inflammatory biomarkers and thus increase the risk of chronic inflammatory diseases like CVD.
Several potential mechanisms may explain the connection between skipping breakfast and chronic inflammation. First, breakfast consumption is positively related to appetite control(Reference Gwin and Leidy35), and subsequently, appetite control affects glycaemic control. Omission of breakfast could impair glycaemic control by interfering with insulin sensitivity and increasing the likelihood of overeating later in the day. An impaired glycaemic control alters postprandial inflammatory responses. When TAG and glucose rise postprandially, neutrophil counts increase codependent with production of pro-inflammatory cytokines and oxidative stress(Reference Farshchi, Taylor and Macdonald36,Reference Klop, Proctor and Mamo37) . Second, breakfast skipping leads to a prolonged overnight fasting period, which may be regarded as a state of stress(Reference Nas, Mirza and Hägele34,Reference Klop, Proctor and Mamo37) . This stress increases adrenergic activity and elevated blood pressure that could lead to increased inflammatory responses, although contradictory data suggested that intermittent fasting with prolonged fasting time could be associated with improved metabolic parameters(Reference Moro, Tinsley and Bianco38,Reference Patterson and Sears39) . Further, habitual breakfast skippers are more likely to make unhealthy lifestyle choices such as smoking, physical inactivity and irregular meal times, compared with regular breakfast eaters(Reference Yokoyama, Onishi and Hosoda7,Reference Chen, Cheng and Liu40) . However, in our study, the inverse association between breakfast consumption and plasma CRP concentration remained, even after adjusting for these lifestyle factors.
The association between breakfast and lower inflammatory status as assessed by CRP concentrations appeared to be stronger among older adults and those who had poor overall diet quality. Ageing and poor diet quality are well-documented risk factors for chronic inflammation and CVD(Reference Singh and Newman41–Reference Giugliano, Ceriello and Esposito43). In the subgroup analysis, stratified by diet quality score, we observed a significant association between breakfast skipping and elevated CRP concentration among individuals with low diet score. This is expectable because poor diet is generally associated with low consumption of anti-inflammatory foods, such as fruit, vegetables and whole grain(Reference Giugliano, Ceriello and Esposito43). Skipping breakfast, together with poor diet quality, may lead to synergistic effects that aggravate inflammatory state(Reference Nas, Mirza and Hägele34,Reference Klop, Proctor and Mamo37) . Although the underlying mechanisms remain unclear, our findings suggest the importance of breakfast consumption in these vulnerable populations. In contrast, insignificant breakfast–CRP relationship was observed among those with high diet quality. This suggests that in individuals with higher diet quality, their lower CRP concentrations compared with breakfast skippers could be attributed largely to the healthier overall diet quality, relative to modest impact of breakfast behaviour.
The ‘breakfast 1–2 times/week’ group appeared to not fit with the overall CRP concentration trends. However, this should be interpreted with caution because the sample size is smaller in this group, relative to other breakfast consumption groups. Thus, this could be due to chance. Another potential explanation is that this group has the youngest age (mean age 41·7 v. 44.4–53 years in other groups) among all breakfast frequency groups. As mentioned previously, ageing is a major determinant of chronic inflammation status(Reference Singh and Newman41,Reference Sanada, Taniyama and Muratsu44) .
Our study has several strengths, including the large sample size and adjustment for a wide range of potential confounders, such as overall diet quality and total energy. Moreover, unlike previous studies on this topic, we also adjusted for several sleep parameters, such as sleep duration, snoring and insomnia in our models because these conditions have been shown to be associated with altered CVD risk and could be relevant to breakfast consumption behaviour(Reference Wang, Liu and Song45–Reference Li, Zhang and Winkelman47). There are also several limitations in the current study. Women (17·7 % of all participants) are under-represented. However, the sample size of women (n 12 458) remains sufficiently large to explore whether the breakfast–inflammation relation could be modified by sex. We used self-report questionnaires to assess breakfast consumption frequency, which could introduce misclassification. Further, previous studies reported that different breakfast foods and breakfast food patterns might have different impacts on cardiometabolic risk(Reference Schwedhelm, Schwingshackl and Agogo48,Reference Adamsson, Reumark and Marklund49) . However, we did not collect information on the specific foods which were consumed during breakfast.
Conclusions
In this large cohort of Chinese adults, we found that habitually skipping breakfast is associated with chronic inflammation, as assessed by CRP concentration, among individuals without CVD, suggesting that the previously observed association between breakfast consumption and altered chronic disease risk could be partially mediated through the effect of regular breakfast consumption on chronic inflammation. These findings should be replicated in future prospective studies with repeated assessment of inflammatory biomarkers (e.g., CRP and IL 6). Future studies should also include detailed information on type and amount of breakfast foods consumed.
Acknowledgements
Acknowledgements: None. Financial support: The current study was supported by grants from the Institute for CyberScience Seed Grant Program, Penn State University and the start-up grant from the college of health and human development and the department of nutritional sciences, Penn State University. Conflict of interest: There are no conflicts of interest. Authorship: Drs S.W. and X.G. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: S.W., K.L.T. and X.G. Acquisition, analysis or interpretation of data: All authors. Drafting of the manuscript: S.Z. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: X.Z. Obtained funding: X.G. Administrative, technical or material support: L.C., X.Z., R.S., H.V.E. and S.W. Supervision: K.L.T., S.W. and X.G. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Ethics Committee of the Kailuan General Hospital. Written informed consent was obtained from all subjects/patients.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020001214








