The diet of vegetarians is generally based on cereals, nuts, fibre, fruits and vegetables and may also include dairy products and eggs(Reference Dwyer1). Several studies have reported that vegetarians are associated with having a favourable lipid profile as evidenced by lower levels of TAG, total cholesterol and LDL-cholesterol(Reference Key, Davey and Appleby2–Reference Lee, Woo and Chen4). In addition, vegetarians are associated with having lower levels of insulin resistance, intramyocellular lipids, blood pressure, BMI and C-reactive protein and higher levels of antioxidants(Reference Szeto, Kwok and Benzie5–Reference Valachovicova, Krajcovicova-Kudlackova and Blazicek7). Collectively, this favourable health profile may explain the lower risk of developing type 2 diabetes and CVD in vegetarians(Reference Key, Davey and Appleby2, Reference Fraser8, Reference Tonstad, Butler and Yan9).
Sex hormones have also been observed to be associated with metabolic complications. Higher levels of testosterone, oestradiol (E2) and dehydroepiandrosterone sulfate (DHEA-s) as well as lower levels of sex hormone-binding globulin (SHBG) have been shown to be associated with the metabolic syndrome and insulin resistance(Reference Polderman, Gooren and Asscheman10–Reference Kalyani, Franco and Dobs13). Furthermore, it had been reported that sex hormones may predict the development of type 2 diabetes(Reference Kalyani, Franco and Dobs13–Reference Ding, Song and Manson15). For example, a recent study showed that lower concentrations of SHBG could strongly predict the risk of type 2 diabetes(Reference Ding, Song and Manson15) in men and women. Kalyani et al. (Reference Kalyani, Franco and Dobs13) indicated that higher levels of testosterone and E2 were associated with the development of type 2 diabetes in post-menopausal women.
Taken together, the lower risk of developing CVD and diabetes in vegetarians may be explained, at least in part, by a favourable sex hormone profile. However, the possible link between the protective profile of vegetarians with sex hormones and metabolic risk factors as well as the risk of type 2 diabetes and CVD is poorly understood. In fact, no data appear to be currently available on the inter-relationships between vegetarians, metabolic risk factors, sex hormones and the risk of developing chronic diseases (i.e. type 2 diabetes). Knowledge of a relationship between vegetarians with sex hormones may help us better understand the risk of disease of an individual and as such provide useful information to health professionals. Therefore, the purpose of the present study was to investigate the sex hormonal and metabolic profiles in vegetarians and compare these with the profiles in omnivores. We hypothesised that vegetarians would present a more favourable sex hormonal and metabolic profile compared with omnivores.
Methods
Subjects
We recruited two groups of women (forty-one omnivores and twenty-one vegetarians) living in the Helsinki area. The participants were voluntarily recruited by newspaper advertisements. Groups in the present study were age-matched. Based on their answers to a questionnaire about the frequency and the type of their physical activity, these women were sedentary or moderately physically active ( ≤ 3 h/week with moderate intensity or ≤ 5 h/week with low intensity). We excluded subjects with a history of any major diseases such as type 2 diabetes and CVD or using drugs such as oral contraceptives, hormonal replacement therapy, or antibiotics. To be included as a regular vegetarian, the women were required to follow this diet for at least 2 years (mean 12 years). Sample blood collections for premenopausal women were performed during the mid-follicular phase of the menstrual cycle (days 5 to 9). The mean day of the menstrual cycle in which the blood collections began was on day 7 ± 1 for the omnivores and day 8 ± 1 for the vegetarians. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethical committee of the Helsinki University Central Hospital. Subjects were initially interviewed by a doctor who explained the study and written informed consent was obtained from all subjects.
Hormones
Plasma samples were collected from fasting subjects. To the heparin plasma samples, we added 0·1 % sodium azide and 0·1 % ascorbic acid, and the samples were then frozen at − 20°C until analysed. As previously described, plasma oestrone (E1) and E2 were determined by RIA after chromatographic separation on an LH-20 column(Reference Adlercreutz, Fotsis and Heikkinen16, Reference Goldin, Adlercreutz and Gorbach17). Plasma testosterone and androstenedione were determined as previously described by Kuoppasalmi et al. (Reference Kuoppasalmi, Naveri and Rehunen18). SHBG was determined as described by Rosner(Reference Rosner19), with slight modifications(Reference Kuoppasalmi20). Free E2 (in percentages and in pmol/l) was calculated from total E2 and SHBG values, and free testosterone (in percentages and in pmol/l) was calculated from testosterone and SHBG values according to Bergink et al. (Reference Bergink, Holma and Pyorala21). DHEA-s was measured by RIA with a commercial kit (Wien Laboratories, Succasunna, NJ, USA). Growth hormone, insulin, apo-A and apo-B were also measured by RIA with commercial kits (CIS International, Gif-sur-Yvette, France). All the methods have been validated with regard to accuracy, sensitivity, specificity and reproducibility.
Total faecal excretion
The total amount of faecal excretion was collected during a 72 h period, kept cold and brought daily to the laboratory by the subject.
Dietary intake
Each subject was provided with a food scale balance and was instructed on how to complete the dietary records. The validity of a 3 d dietary record to estimate dietary intake in adults without cognitive impairments has been confirmed(Reference Luhrmann, Herbert and Gaster22). Dietary analyses were completed by a nutritionist using the 1983 version of a coding system from the Department of Nutrition (University of Helsinki) for total, lipid, carbohydrate, protein, cholesterol, MUFA, PUFA, starch, sucrose, lactose, Ca and alcohol intake. For fibre data, we used Southgate tables(Reference Paul and Southgate23), and for some typical Finnish foods (i.e. rye products), we used the data given by manufacturers. Fibre values are presented separately for cereal, vegetables, berries/fruits and total.
Physical activity level
The level of physical activity was evaluated with a frequency questionnaire (how long and how many sessions per week). We categorised women as physically inactive if they practised physical activity three or fewer times or ≤ 3 h/week. The level of physical activity has been considered low or moderate when the intensity was between 0 and 8 kcal/min (0–33 kJ/min) or less than 1000 kcal/week (4184 kJ/week).
Statistical analysis
Data are expressed as the mean values and standard deviations. An independent t test was used for the comparison of variables between omnivores and vegetarians as well as between pre- and post-menopausal vegetarian women. Moreover, using a univariate general linear model analysis, we statistically controlled for BMI to verify if it would abolish differences in SHBG, free E2, free testosterone, DHEA-s, total faecal excretion per 72 h, apo-B and apo-A between groups. A χ2 test was used to compare menopause status between the two groups. Pearson correlations were performed to examine the relationship between SHGB with free E2, free testosterone, DHEA-s, total faecal excretion per 72 h, apo-B apo-A, BMI, total fibre and muscle mass. Finally, a stepwise multi-linear regression analysis was performed to identify predictors of SHBG. Independent variables considered in the final model for SHBG were total fibre, BMI, apo-A, apo-B, total faecal excretion per 72 h and free E2. We chose to determine factors predicting SHBG because a recent study showed that lower concentrations of SHBG could strongly predict the risk of type 2 diabetes in men and women(Reference Ding, Song and Manson15). Therefore, we find it timely to examine predictors of SHBG. Statistical analysis was performed using SPSS 15 for Windows (SPSS, Inc., Chicago, IL, USA). Significance was accepted at P < 0·05.
Results
Physical and metabolic characteristics of omnivores and vegetarians are presented in Table 1. Both groups were comparable for age, menopause status, age of menopause, height, physical activity, insulin, NEFA and total urinary excretion per 72 h. Body weight, BMI, apo-B, and the apo-B:apo-A ratio were significantly lower in vegetarians (P < 0·05), whereas apo-A and total faecal excretion per 72 h were significantly higher in vegetarians (P < 0·01).
Table 1 Physical and metabolic characteristics of omnivores and vegetarians
(Mean values and standard deviations)
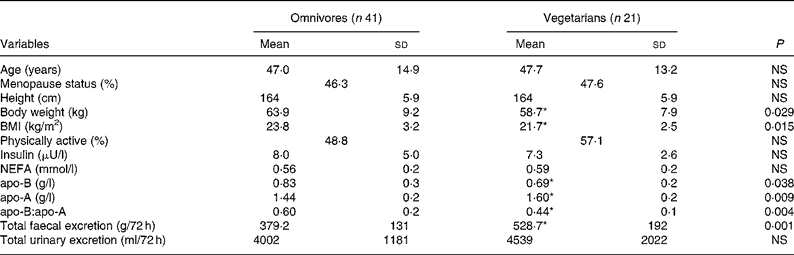
* Mean value was significantly different from that of omnivores (P < 0·05).
Plasma steroid hormones are shown in Table 2. SHBG was significantly higher in vegetarians (P < 0·01), whereas free E2, free testosterone and DHEA-s were significantly lower (P < 0·05). No other differences were noted.
Table 2 Plasma steroid hormones in omnivores and vegetarians
(Mean values and standard deviations)
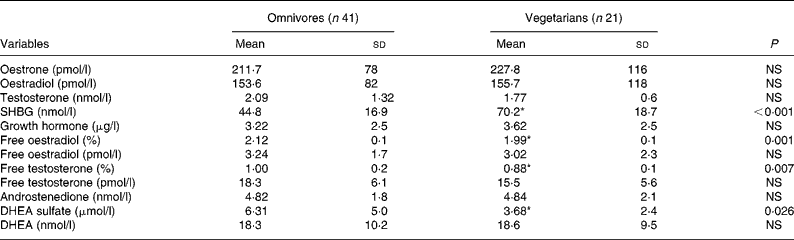
SHBG, sex hormone-binding-globulin; DHEA, dehydroepiandrosterone.
* Mean value was significantly different from that of omnivores (P < 0·05).
Dietary characteristics of omnivores and vegetarians are described in Table 3. Energy intake was comparable between both groups. Carbohydrates, total fibre, vegetable fibre, berry/fruit fibre and total fibre/kg body weight were significantly higher in vegetarians, whereas protein, total fat, MUFA and cholesterol were significantly lower in vegetarians. No other differences were noted.
Table 3 Dietary characteristics of omnivores and vegetarians
(Mean values and standard deviations)
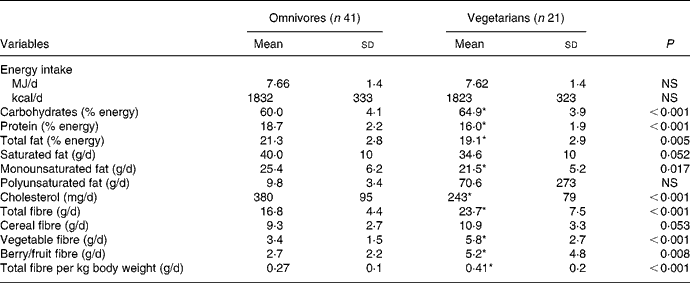
* Mean value was significantly different from that of omnivores (P < 0·05).
When statistically controlling for BMI, significant differences in SHBG, total faecal excretion per 72 h, apo-A, free E2, free testosterone and DHEA-s levels between the groups persisted. When the apo-B:apo-A ratio and apo-B levels were statistically controlled for BMI, statistical significance was abolished between the groups. Furthermore, significant correlations were observed between SHBG with total faecal excretion per 72 h, free E2, free testosterone, apo-A, apo-B, apo-B:apo-A ratio and total fibre.
In a sub-analysis, using all vegetarians in our cohort (n 21), we compared the hormonal, metabolic and dietary profiles of pre- (n 11) and post- (n 10) menopausal women separately (using Mann–Whitney analysis). Results show that no differences were observed for hormonal (except for oestrogen-related hormones), metabolic and dietary profiles between pre- and post-menopausal vegetarian women. However, the present results should be considered preliminary due to the low number of subjects, but they may hopefully stimulate interest in the need for greater participant characterisation in research protocols. Moreover, we analysed the sex hormone levels in the plasma for pre- (n 22) and post-menopausal (n 19) omnivores and found that oestrone, E2, free E2, androstenedione, SHBG, DHEA-s and DHEA levels were significantly lower in post-menopausal omnivores. In addition, no differences in testosterone and free testosterone as well as growth hormone were observed between the groups. It should be noted that the age of menopause for vegetarians and omnivores was 50 (sd 2) v. 50 (sd 4) years, respectively, and the age of the post-menopausal women at the start of the study for vegetarians and omnivores was 60 (sd 4) v. 61 (sd 7) years, respectively. Therefore, the mean length of menopause for vegetarians and omnivores was 10 v. 11 years, respectively (NS).
Finally, we performed a stepwise regression analysis to identify independent predictors of SHBG. Table 4 illustrates the summary of the model. The present results show that total fibre and BMI were independent predictors of SHBG, collectively explaining 25·0 % of the variance (P < 0·01).
Table 4 Stepwise regression analysis regarding independent predictors of sex hormone-binding globulin (SHBG)

Discussion
Several studies have reported that vegetarians are associated with a favourable metabolic profile, which could reduce the risk of developing type 2 diabetes and CVD(Reference Key, Davey and Appleby2, Reference Goff, Bell and So6, Reference Tonstad, Butler and Yan9). To add to the body of literature, we attempted to provide new information on metabolic risk factors such as sex hormones that may characterise the profile of vegetarians in pre- and post-menopausal women.
We hypothesised that vegetarians would present a more favourable sex hormonal and metabolic profile compared with omnivores. Results from the present study support our hypothesis. That is, we found that SHBG levels were significantly higher by 56·7 % in vegetarian women compared with omnivore women. Higher concentrations of SHBG have been recently shown to be associated with a favourable metabolic profile as well as reducing the risk of type 2 diabetes(Reference Onat, Hergenc and Karabulut11, Reference Ding, Song and Manson15). In addition, vegetarians had lower levels of free E2, free testosterone and DHEA-s. Furthermore, vegetarians showed higher levels of apo-A, total faecal excretion per 72 h and total fibre intake as well as lower levels of apo-B, BMI and total fat intake. Moreover, several studies have shown that higher fibre intake may be associated with higher total faecal excretion mass and a more favourable metabolic profile(Reference Jenkins, Kendall and Popovich24–Reference McIntosh, Noakes and Royle26). In the present study we observed higher levels of total faecal excretion per 72 h and total fibre intake in vegetarians. Interestingly, after controlling for BMI, all metabolic and hormonal differences between the groups remained significant. This suggests that the differences between the groups were independent of BMI. However, differences in apo-A levels between the groups were abolished after controlling for BMI, suggesting that BMI may be a potential mediating factor.
Collectively, these results suggest that vegetarians display favourable metabolic, sex hormonal and dietary profiles compared with omnivores in our cohort, which may be associated with a decreased risk of type 2 diabetes and CVD. These findings confirm and extend the results of several other studies, which also showed that vegetarians are associated with a favourable metabolic and dietary profile(Reference Key, Davey and Appleby2, Reference Lee, Woo and Chen4, Reference Goff, Bell and So6, Reference Onat, Hergenc and Karabulut11, Reference Laaksonen, Niskanen and Punnonen27). These results also suggest that multiple factors could be implicated in the healthy profile of vegetarian women. Therefore, studies characterising vegetarians may want to consider several outcome measures in different domains in order to have a complete understanding of vegetarians.
In a sub-analysis, using all vegetarians in our cohort, we compared the hormonal, metabolic and dietary profiles of pre- and post-menopausal women separately. Interestingly, results in the present study show that no differences were observed for hormonal (except for oestrogen-related hormones), metabolic and dietary profiles between pre- and post-menopausal vegetarian women. This suggests that the protective profile of vegetarians could be sustained after the menopause. This finding is important since the menopause could be associated with an increased risk of CVD(Reference Gorodeski28, Reference Knopp29).
Results from the logistic regression analysis showed that total fibre intake and BMI were independent predictors of SHBG in our cohort. This suggests that higher levels of total fibre intake and a lower BMI may be associated with higher levels of SHBG. In support of this idea, we showed that total fibre intake explained 15·2 % of the variation in SHBG levels in our cohort, which accounted for the greatest source of unique variance. Therefore, high fibre intake could contribute to high levels of SHBG, which may lead to a lower risk of developing type 2 diabetes. This would be a testable hypothesis that warrants further investigation.
The present study has several limitations. First, our cohort is only composed of non-diabetic pre- and post-menopausal women. Therefore, our findings are limited to this population. Second, we used a cross-sectional approach, which does not allow us to conclude any causal associations between SHBG levels and total fibre intake in our cohort. Finally, the present study lacks detailed body composition measures and dietary intake may be under- and mis-reported. Nonetheless, the present results are strengthened by studying a well-characterised cohort.
In conclusion, results of the present study indicate that pre- and post-menopausal vegetarians present favourable hormonal, metabolic and dietary profiles, in particular, higher concentrations of SHBG, which may be associated with higher levels of total fibre intake. This favourable dietary, hormonal and metabolic profile may explain, at least in part, the lower risk of developing type 2 diabetes in vegetarians.
Acknowledgements
The present study was supported by the Medical Research Council in the Academy of Finland, the Sigrid Jusélius Foundation and Samfundet Folkhälsan. M. A.-L. was supported by the Canadian Institutes of Health Research (CIHR). A. D. K. was supported by the Fonds de la Recherche en Santé du Québec (FRSQ).
A. D. K. contributed to the analysis and interpretation of data as well as to the writing of the manuscript. A. F. and M.-E. F. were involved in the writing of the manuscript and provided significant advice. H. A. designed the experiment and provided significant advice. M. A.-L. was involved in the collection of data and provided significant advice and revisions.
The authors report no conflict of interest.






