Introduction
Chicken coccidiosis is a serious protozoan disease caused by Eimeria spp. parasitizing the intestines. It is estimated that the global poultry industry incurs losses of up to $14 billion annually due to coccidiosis (Blake et al., Reference Blake, Knox, Dehaeck, Huntington, Rathinam, Ravipati, Ayoade, Gilbert, Adebambo, Jatau, Raman, Parker, Rushton and Tomley2020). Even low doses of Eimeria oocysts can cause intestinal pathology, alter intestinal morphology and impair intestinal barrier function and development (Gelinas et al., Reference Gelinas, Sudan, Patterson, Li, Huyben, Barta and Kiarie2024). Eimeria infection is widely recognized as a primary risk factor for necrotic enteritis caused by Clostridium perfringens (Blake et al., Reference Blake, Marugan-Hernandez and Tomley2021). In addition, infection with Eimeria spp. leads to intestinal flora imbalance, which affects the structure and diversity of microbial communities as well as the transmission of foodborne zoonotic pathogens, resulting in losses that are difficult to quantify.
For a long time, the large-scale use of anti-coccidial drugs has been an efficient method for controlling coccidiosis (Peek and Landman, Reference Peek and Landman2011). Two main types of anti-coccidial drugs are currently used: chemically synthesized drugs (including diclazuril and decoquinate) and polyether ionophores (including maduramicin and salinomycin). Maduramicin and diclazuril are widely used to control coccidiosis with remarkable efficacy. Maduramicin interferes with the permeability of the coccidia cell membrane to affect the monovalent cations. The coccidia use energy to exclude the large amount of Na+ entering the cell, and finally, the coccidia die due to energy depletion (Kant et al., Reference Kant, Singh, Verma, Bais, Parmar, Gopal and Gupta2013). Diclazuril affects the nucleic acid synthesis of coccidia and prevents the differentiation of schizonts and microgametocytes (McDougald et al., Reference McDougald, Mathis and Seibert1990; Hunyadi et al., Reference Hunyadi, Papich and Pusterla2015). However, the widespread use of drugs over time has created a severe problem of drug resistance. Since the isolation of sulfonamide-resistant strains from chickens on farms in the United States in 1954, strains resistant to almost all anticoccidial drugs have been isolated. Furthermore, multiple cross-resistant strains have appeared (Abbas et al., Reference Abbas, Iqbal, Blake, Khan and Saleemi2011; Zhang et al., Reference Zhang, Zhang, Ren, Si, Song, Liu, Suo and Hu2023). Drug resistance has become the most important obstacle in the prevention and control of coccidiosis.
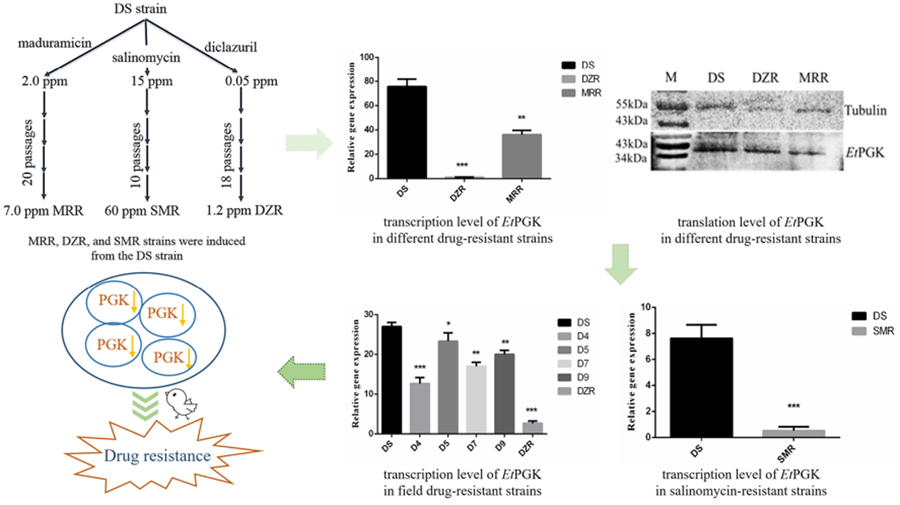
To study the molecular mechanism of drug resistance, our laboratory successfully induced and obtained diclazuril-resistant (DZR) and maduramicin-resistant (MRR) strains based on a drug-sensitive (DS) strain of Eimeria tenella using a drug concentration-increasing method. The obtained DZR strain was fully resistant to 1.2 ppm diclazuril, while the MRR strain was only fully resistant to 7.0 ppm maduramicin (Han et al., Reference Han, Zhao, Chen and Huang2004). We then used RNA-sequencing to screen genes differentially expressed among the DZR, MRR and DS strains of the same genetic background. The results showed differential expression of phosphoglycerate kinase of E. tenella (EtPGK), which was significantly downregulated in the DZR and MRR strains compared with the DS strain (Xie et al., Reference Xie, Huang, Xu, Zhao, Zhu, Zhao, Dong and Han2020).
Phosphoglycerate kinase (PGK) is an essential enzyme in the second phase of the aerobic glycolysis pathway. It catalyses the conversion of 1,3-bisphosphoglycerate to 3-phosphoglycerate, during which 1 molecule of ADP is consumed to produce 1 molecule of ATP. If the reverse reaction occurs, 1 molecule of ADP is formed (He et al., Reference He, Luo, Zhang, Wang, Zhang, Li, Ejaz and Liang2019). In addition to regulating cellular metabolism, PGK is involved in a wide range of biological activities, including DNA repair, autophagy and angiogenesis (Banks et al., Reference Banks, Blake, Evans, Haser, Rice, Hardy, Merrett and Phillips1979). Abnormal levels of PGK expression have been associated with disease development. For example, PGK deficiency is related to neurological damage, Parkinson's disease and myopathy (Echaniz-Laguna et al., Reference Echaniz-Laguna, Nadjar, Béhin, Biancalana, Piraud, Malfatti and Laforêt2019; Hogrel et al., Reference Hogrel, Ledoux and Béhin2019). PGK is a drug resistance-related protein in cancer cells, and high expression of PGK protein may promote drug resistance through complex molecular mechanisms (He et al., Reference He, Luo, Zhang, Wang, Zhang, Li, Ejaz and Liang2019). PGK has been studied in parasites such as Plasmodium falciparum, Trypanosoma cruzi and Leishmania major but has not been reported in Eimeria.
In this study, EtPGK was cloned and successfully expressed. We evaluated the differential expression of EtPGK in drug-resistant strains and the DS strain. In addition, we analysed the expression levels and distribution of EtPGK at different developmental stages of E. tenella.
Materials and methods
Animals and parasites
The yellow chickens used in the experiment were supplied by a farm (Shanghai, China), and the New Zealand rabbits were purchased from the Jiagan Biology Company (Shanghai, China). All animals were reared in a coccidia-free environment with free access to feed and water.
Eimeria tenella DS strain was isolated from a farm in Shanghai (Resource Number CAAS21111601) and kept in our laboratory, maintained by passage every 3 months in susceptible 14-day-old yellow-feathered chickens (Huang et al., Reference Huang, Wu, Shi and Chen1993). Our laboratory-induced MRR, DZR and salinomycin-resistant (SMR) strains were each only resistant to 7.0 ppm maduramicin, 1.2 ppm diclazuril and 60 ppm salinomycin, respectively (Han et al., Reference Han, Zhao, Chen and Huang2004; Wang et al., Reference Wang, Zhao, Zhu, Huang, Dong, Wang, Yu, Yu, Liang and Han2019). Three drug-resistant strains were also passaged by infected chickens feeding corresponding drugs.
The unsporulated oocysts (UO) were collected and purified using standard procedures, and sporulated oocysts (SO) were formed after oxidation of UO at 28°C (Han et al., Reference Han, Lin, Zhao, Dong, Jiang, Xu, Zhu and Huang2010). Sporozoites (SZ) were collected from SO in vitro (Miska et al., Reference Miska, Fetterer and Barffeld2004). The second-generation merozoites (SM) were collected from the cecum infected with E. tenella 120 h after inoculation (Zhou et al., Reference Zhou, Wang, Wang, Zhang, Zhang and Xue2010).
The single-oocyst method was used to isolate the field diclazuril-resistant strains (D4, D5, D7 and D9). Their resistance to 1.0 ppm diclazuril was demonstrated using drug sensitivity experiments in chickens (Khalafalla and Daugschies, Reference Khalafalla and Daugschies2010).
Amplification, cloning and sequencing of EtPGK
Total RNA was extracted from SO of E. tenella DS strain using TRIzol reagent (TaKaRa, Tokyo, Japan). The RNA was treated with DNase I and transcribed into cDNA using a reverse transcriptase kit (Invitrogen, Carlsbad, CA, USA) and oligo (dT) primer. cDNA was then used as the amplification template.
The ORF of EtPGK (ToxoDB Accession No.: ETH_00015140) was amplified by PCR using the primers 5′-GCGGAATTCATGCGCGTGGACTTCAACGTGCCG-3′ (sense) and 5′-GCGCTCGAGATTCTCCAGGAGCTCCAGAGAG-3′ (antisense), including EcoR I and Xho I restriction sites. Amplified products were analysed and purified by 1% agarose gel electrophoresis (Qiagen, Dusseldorf, Germany) and subcloned into the pGEM-T-easy vector (Promega, Madison, WI, USA). The positive clone for recombinant plasmid was identified by DNA sequence.
The full-length cDNA sequence of EtPGK was analysed using BLAST program of the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/BLAST/). SignalP (https://www.cbs.dtu.dk/service s/SignalP) was used to predict signal peptide, Motif Scan (https://hits.isb-sib) was used to predict protein motifs, and the transmembrane motif was determined by TMHMM (https://www.cbs.dtu.dk/services/T MHMM-2.0/).
Quantitative real-time PCR (qRT-PCR)
The mRNA expression of EtPGK at different developmental stages (UO, SO, SZ and SM) of E. tenella DS strain was determined by real-time quantitative PCR (qRT-PCR). The qRT-PCR was performed using SYBR1 Green I dye method. Total RNA from UO, SO, SZ and SM was extracted as described above, and genomic DNA was removed using RNeasy Mini Kits (Qiagen). cDNA was synthesized from total RNA of different developmental stages using SuperScript II reverse transcriptase (Invitrogen) and random primers (Invitrogen). The EtPGK primers used for qRT-PCR were 5ʹ-GCTGCTGACCTGGCTGCTGACCTGCTGCTGCT-3′ (sense) and 5ʹ-ACGTGCCGCTCAAAGACGGG-3′ (antisense). The 18S rRNA housekeeping gene (GenBank number: EF122251) of E. tenella was used as an internal control with primers 5ʹ-TGTAGTGGAGTCTTGTGGATTC-3′ (sense) and 5ʹ-CCTGCTGCC TTCCTTAGATG-3′ (antisense). Relative expression of EtPGK was measured using the 2-ΔΔCt method (Livak and Schmittgen, Reference Livak and Schmittgen2001). Reactions were performed in triplicate and experiments were repeated 3 times.
We also compared the transcription levels of EtPGK in SO of DZR, MRR, SMR and DS strains using qRT-PCR. The transcription levels of DZR strains resistant to different concentrations of diclazuril (0.2, 0.5, 0.8 and 1.0 ppm) and MRR strains resistant to different concentrations of maduramicin (3 and 5 ppm) were analysed compared with DS strain. The transcription levels of EtPGK in the field DZR strains (D4, D5, D7 and D9) were also detected using qRT-PCR.
Protein expression and polyclonal antibody preparation
The expression vector pGEX-4T-1 (Novagen, Darmstadt, Germany) and the recombinant plasmid pGEM-T-EtPGK were double-digested with EcoR I and Xho I, and then ligated to construct the recombinant expression plasmid pGEX-4 T-EtPGK. pGEX-4 T-EtPGK plasmid was transformed into Escherichia coli BL21 (DE3) (Tiangen, Beijing, China) and induced expressed recombinant protein (rEtPGK) with 1.0 mm isopropyl β-D-1-thiogalactopyranoside (IPTG; Sigma-Aldrich, St. Louis, MO, USA). The expression form of rEtPGK was determined by 12% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) analysis after sonication. rEtPGK was purified from SDS-PAGE gel strips (Richard, Reference Richard2009). The quality of purified rEtPGK was identified by 12% SDS-PAGE, and the concentration of purified protein was determined by BCA protein detection reagent (Beyotime, Haimen, China).
Three-month-old New Zealand rabbits were immunized subcutaneously with purified rEtPGK (200 μg per rabbit) 5 times at 1-week intervals. Freund's complete adjuvant (Sigma-Aldrich) and rEtPGK protein were mixed at a ratio of 1:1 for the first immunization, and Freund's incomplete adjuvant was used for subsequent immunizations (Sigma-Aldrich). Polyclonal antibodies to rEtPGK were collected 7 days after the last immunization. Serum collected before protein injection was used as a negative control.
Western blot
The expression levels of EtPGK in UO, SO, SZ and SM of E. tenella DS strain were analysed by western blot. Total proteins from 4 stages were prepared using cell lysis buffer. The concentration of protein was determined using a BCA protein assay kit (Beyotime). The same quantity of proteins (20 μg) was separated by 12% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, Burlington, MA, USA). The membranes were blocked with 5% (w/v) skimmed milk in phosphate-buffered saline (PBS). The primary antibody was anti-rEtPGK polyclonal rabbit antibody, while α-tubulin mouse antibody (Beyotime) was used as a control. HRP-conjugated goat anti-rabbit IgG or HRP-conjugated goat anti-mouse IgG (H + L) was used as secondary antibody. The bands were detected with a Supersignal West Pico Plus chemiluminescent substrate kit (Thermo Fisher, Waltham, MA, USA) using Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
Using the same method, the translation level in SO of EtPGK among DS, DZR and MRR strains was determined using rEtPGK-immunised rabbit serum and α-tubulin mouse antibody as primary antibody by western blot.
Localization of EtPGK by indirect immunofluorescence
The freshly extracted SZ and SM were evenly applied to the glass slides and then placed in the 6-well plate to dry. The glass slides were placed into the 6-well plate and subsequently inoculated with DF-1 cells (3 × 105 per well) and cultured for 24 h. Purified SZ (9 × 105 cells per well) were added to invade and develop in the cells. The glass slides were removed at various time points after inoculation, then washed and air-dried. The cells were fixed in 4% paraformaldehyde solution for 30 min, permeabilized with 1% Triton X-100 for 15 min and then blocked with 2% (w/v) bovine serum albumin at 37°C for 2 h. Then the rabbit anti-rEtPGK was added and incubated at 37°C for 2 h. Slides were then incubated with goat anti-rabbit IgG fluorescein isothiocyanate-conjugated antibody (Sigma-Aldrich) at 37°C for 40 min. Nuclei of parasites and cells were stained with 10 mg mL−1 40, 6-diamidino-2-phenylindole (Beyotime) for 15 min at room temperature. After each step, glass slides were washed 5 times with PBST. Slides were treated with 50 μL Fluoromount Aqueous Mounting Medium (Sigma-Aldrich) and observed under a fluorescence microscope (Olympus, Tokyo, Japan).
Sporozoite invasion inhibition assay
Referring to the method of Wilson et al. (Reference Wilson, Goodman, Sleebs, Weiss, de Jong, Angrisano, Langer, Baum, Crabb, Gilson, McFadden and Beeson and2015), an invasion assay was performed to test the effect of rabbit anti-rEtPGK polyclonal antibody on the invasion of DF-1 cells by SZ. The 2 × 105 DF-1 cells were added in advance to a 24-well plate and cultured for 24 h. Freshly extracted SZ of DS strain were labelled with carboxyfluorescein diacetate succinimidyl ester (Beyotime), then incubated with different concentrations of purified rabbit IgG against rEtPGK (50, 100, 200, 300 and 400 μg mL−1), with the same concentration of normal rabbit serum IgG as a control, and a blank group was set at the same time. After incubation at 41°C for 2 h, SZ were centrifugated and resuspended in complete culture medium. Then SZ (6 × 105 per well) were added to the 24-well plate containing DF-1 cells, and cultured for 6 h at 41°C and 5% CO2. Uninvaded SZ were washed by PBS, and the invasion rate of SZ into DF-1 cells was detected by flow cytometry.
Enzyme activity
Whole proteins of fresh DS, DZR and MRR strains of SO (1 × 107) were obtained by sonication on ice. The concentration of protein was determined using a BCA protein assay kit (Beyotime). The protein concentration was diluted to 0.5 mg mL−1 with RNAase-free water and determined spectrophotometrically using the EtPGK Enzyme Activity Assay Kit (Thermo Scientific). Then, 50 μL of reaction buffer was added to each well and mixed with the substrate, and 50 μL of 0.5 mg mL−1 DS, DZR or MRR protein was added. The results were observed after 20 min at 340 nm.
Results
Characterization of the EtPGK sequence
The ORF sequence of EtPGK we amplified was 100% homologous to E. tenella phosphoglycerate kinase (GenBank: XM_013373443.1) and 91% homologous to Eimeria necatrix phosphoglycerate kinase (GenBank: XM_013373443.1). Analysis of the EtPGK nucleotide sequence showed that the EtPGK sequence was 1206 bp, encoding 401 amino acid residues, with an isoelectric point of 7.01 and a predicted molecular weight of 42.3 kDa. There was no signal peptide or transmembrane region. The protein encoded by this gene contained 4 casein kinase II phosphorylation sites (78–81, 213–216, 240–243, 362–365); 4 N-myristoylation sites (221–226, 290–295, 356–361, 379–384); 5 protein kinases C phosphorylation sites (26–28, 56–58, 74–76, 227–229, 399–401); an alanine-rich region (331–351); and a Pumilio RNA-binding repeat sequence (230–267) (Fig. 1).

Figure 1. Bioinformatic analysis of EtPGK. Black spots: Pumilio RNA-binding repeat profile; box: alanine-rich region profile; underline: protein kinase C phosphorylation site; yellow: N-myristoylation site; red: casein kinase II phosphorylation site; *stop codon.
Expression and purification of recombinant EtPGK
We successfully expressed EtPGK in E. coli BL21 as a GST-tagged fusion protein. SDS-PAGE showed that rEtPGK was expressed mainly in the inclusion bodies (Fig. 2A). Then we purified recombinant protein by cutting gel purification. The molecular mass of the rEtPGK fused to the GST-tag (26 KDa) was found to be approximately 68 kDa (Fig. 2B).

Figure 2. Expression of recombinant protein EtPGK. (A) Expression form of recombinant protein. M: protein molecular weight marker; 1: the precipitated; 2: the supernatant. (B) Purification of recombinant protein.
EtPGK transcription and translation at different developmental stages of the DS strains
Using 18s rRNA as an internal reference gene, qRT-PCR was used to detect mRNA transcription levels of EtPGK at different developmental stages of E. tenella DS strains. The results showed that the mRNA transcription levels of EtPGK was highest in SO, followed by SZ, and lowest in UO and SM (Fig. 3A). The translation levels of EtPGK were examined by western blot using α-tubulin as a control. According to Fig. 3B, the translation level of EtPGK was higher in SO than other 3 stages, and lowest in UO.

Figure 3. Transcription and translation levels of EtPGK in different developmental stages of E. tenella. (A) Transcription levels of EtPGK. (B) Translation levels of EtPGK. UO, unsporulated oocyst; SO, sporulated oocyst; SZ, sporozoite; SM, second generation merozoite. a, b and c indicate significant differences (P < 0.05) between groups.
Differences in transcription and translation levels of EtPGK between sensitive and resistant strains
The mRNA transcription levels of EtPGK in DS, DZR and MRR strains of E. tenella were detected by qRT-PCR. Using 18S rRNA as an internal reference gene, the transcription levels of EtPGK were significantly downregulated in both resistant strains compared with DS strain (Fig. 4A). Whole proteins of E. tenalla of DS, DZR and MRR strains were collected and analysed by western blot using α-tubulin as a control. The results showed that translation level of EtPGK was significantly downregulated in the DZR and MRR strains (Fig. 4B).

Figure 4. Expression levels of EtPGK in drug-sensitive (DS), diclazuril-resistant (DZR) and maduramicin-resistant (MRR) strains. (A) Transcription levels of EtPGK. (B) Translation levels of EtPGK. *P < 0.05; **P < 0.01; ***P < 0.001.
It was also found that the mRNA transcription levels of EtPGK decreased with increasing drug concentrations of maduramicin and diclazuril. The transcription levels of different concentrations of DZR and MRR strains were significantly downregulated compared with the DS strain (Fig. 5).

Figure 5. Transcription levels of EtPGK in drug-resistant strains at different concentrations. ppm: mg kg−1; (A) different concentrations of diclazuril; (B) different concentrations of maduramicin. *P < 0.05; ***P < 0.001.
We analysed mRNA transcription level of EtPGK in the field isolated diclazuril-resistant strains (D4, D5, D7 and D9) using qRT-PCR. The result showed that the transcription level of EtPGK was also downregulated in the field diclazuril-resistant strains compared with DS strain (Fig. 6A). However, they still have a gap compared with the laboratory-induced DZR strain that is completely resistant to 1.2 ppm diclazuril.

Figure 6. EtPGK transcript levels in other drug-resistant strains. (A) Transcription levels of EtPGK in diclazuril-resistant strains obtained from the field; D4–D9, 4 field isolated diclazuril-resistant strains. (B) Transcription levels of EtPGK in salinomycin-resistant (SMR) strain. *P < 0.05; **P < 0.01; ***P < 0.001.
Using qRT-PCR, we also compared the transcription levels of EtPGK in the SO of DS and SMR strains. It was found that the transcription level of EtPGK in the SMR strain was significantly downregulated compared with the DS strain (Fig. 6B).
Immunofluorescence localization of EtPGK
The distribution of EtPGK at different developmental stages of E. tenella was analysed by indirect immunofluorescence localization. It was observed that EtPGK was mainly located on the surface and in the cytoplasm of the SZ with the exception of the refractile body (Fig. 7A) and SM (Fig. 7B). During the invasion of DF-1 cells by SZ to develop into schizonts, EtPGK was distributed on the surface and cytoplasm of schizonts, and the fluorescence intensity was enhanced (Fig. 7C–F).

Figure 7. Distribution and localization of EtPGK at different development stages. (A) Sporozoites (SZ) in PBS; (B) second-generation merozoites (SM) in PBS; (C) sporozoites at 12 h p.i; (D) immature schizonts (iSc) at 48 h p.i; (E) iScat 62 h p.i.
Inhibition of E. tenella SZ invasion by antibodies against rEtPGK
Invasion inhibition assay was used to determine the effect of rabbit anti-rEtPGK polyclonal antibody on the invasion of SZ of DS strain into DF-1 cells. It was found that the inhibition rate was positively correlated with the antibody concentration, and the inhibition rate of rabbit anti-rEtPGK polyclonal antibody at a concentration of 400 μg mL−1 reached 30%. Meanwhile, rabbit negative IgG showed no significant inhibition of SZ (Fig. 8).

Figure 8. Effect of anti-rEtPGK polyclonal antibody on sporozoite invasion.
Enzyme activity
The enzyme activities of DS, DZR and MRR were analysed using PGK enzyme activity assay kit. The result showed that compared with DS strain, the enzyme activity of EtPGK was significantly reduced in the SO of DZR strain. There was no significant difference in EtPGK enzyme activity between MRR and DS strain (Fig. 9).

Figure 9. Enzyme activity of EtPGK. **P < 0.01.
Discussion
PGK is a glycolytic enzyme that is highly conserved in various organisms and catalyses one of the 2 ATP-generating reactions in the glycolysis pathway. PGK was classified as a moonlighting protein because it is also involved in several different functions separate and distinct from energy metabolism, including pathogenesis, interaction with nucleic acids, tumorigenesis and progression, cell death and viral replication (Rojas-Pirela et al., Reference Rojas-Pirela, Andrade-Alviárez, Rojas, Kemmerling, Cáceres, Michels, Concepción and Quiñones2020). Our results showed that the transcription and translation levels of EtPGK were highest in SO and gradually decreased in SZ and SM. Compared with the other stage, the SO was produced in a relatively oxygen-rich environment in vitro. Therefore, we speculated that the change in the expression level of EtPGK may be related to changes in the environment, such as temperature and oxygen content. Li et al. (Reference Li, Zhang and Yao2019) found that PGK was widely expressed in most tissues of Litopenaeus vannamei, with the highest expression levels in the muscle, where oxygen demand is the highest, followed by the heart and brain. The lowest expression levels were in the small intestine. Some studies have found that PGK contains a Per-Arnt-Sim (PAS) domain. PAS domain-containing proteins are major participants in the adaptation of prokaryotes and eukaryotes to environmental stimuli and can act as input modules to sense oxygen, redox potential and other stimuli (Taylor and Zhulin, Reference Taylor and Zhulin1999; Adhikari et al., Reference Adhikari, Biswas, Mukherjee, Das and Adak2019).
We also found that PGK was distributed on the surface and in the cytoplasm of E. tenella through indirect immunofluorescence localization. As a multifunctional protein, its distribution in different parts of the parasite may play different functions. PGK in Schistosoma japonicum (SjPGK) was found to be distributed not only in the tegument but also in other tissues. Using qRT-PCR, SjPGK was found to be expressed at all developmental stages studied (Hong et al., Reference Hong, Huang, Yang, Cao, Han, Zhang, Han, Fu, Zhu, Lu, Li and Lin2015). Based on its distribution on the parasite surface, we speculated that EtPGK might be related to the invasion of E. tenella into host cells. Invasion inhibition assays found that SZ incubated with rabbit anti-rEtPGK polyclonal antibody serum had a reduced ability to invade DF-1 cells. We hypothesized that EtPGK plays an important role in E. tenella invasion of cells. Studies have found that PGK is a major outer surface protein of group B streptococci, and PGK can bind to the host's α-actinin to facilitate streptococcal invasion of host epithelial cells (Burnham et al., Reference Burnham, Shokoples and Tyrrell2005). As a glycolytic enzyme, EtPGK may provide energy for SZ and SM to invade intestinal epithelial cells. Hong et al. (Reference Hong, Shin, Kang and Ryu2003) found that miracidia in the eggs of Clonorchis sinensis may accumulate PGK to generate the energy required for post-hatching movement. In addition, we speculated whether EtPGK would be secreted extracellularly by the parasite to perform its function. We verified by secretion experiments that EtPGK was not a secreted protein (data not shown).
Chicken coccidia, such as Plasmodium and Toxoplasma, are apicomplexan protozoan parasites. These exclusively intracellular parasites develop in a hypoxic or anaerobic environment. Therefore, some genes involved in the tricarboxylic acid cycle and β-oxidation pathway were lost in the process of evolution (Jacot et al., Reference Jacot, Waller, Soldati-Favre, MacPherson and MacRae2016). Studies found that the mitochondria of apicomplexan protozoan parasites lack type I NADH dehydrogenase and the enzymes and transporters required for fatty acid β-oxidation (Mogi and Kita, Reference Mogi and Kita2010; Danne et al., Reference Danne, Gornik, Macrae, McConville and Waller2013). Therefore, we speculated that glycolysis would be the main way in which the parasite would obtain energy when the parasite is in the host. Previous studies showed that the transcription and translation levels of enolase 2, glyceraldehyde-3-phosphate dehydrogenase and malate dehydrogenase of E. tenella in glycolysis pathway in drug-resistant strains were significantly higher than those in the DS strain, as verified by the results of RNA-seq (Chen et al., Reference Chen, Huang, Zhao, Dong, Zhu, Zhao, Lv, Yan and Han2018; Huang et al., Reference Huang, Zhu, Chen, Zhao, Dong, Huang, Yao, Liu, Yu and Han2022; Yu et al., Reference Yu, Huang, Wang, Dong, Zhao, Zhu, Huang and Han2023). A study of drug resistance of Plasmodium also found that changes in protein expression levels were closely related to the emergence of drug resistance. Birnbaum et al. (Reference Birnbaum, Scharf, Schmidt, Jonscher, Hoeijmakers, Flemming, Toenhake, Schmitt, Sabitzki, Bergmann, Fröhlke, Mesén-Ramírez, Blancke Soares, Herrmann, Bártfai and Spielmann2020) found that decreased expression of Pfkelch13 protein and its interaction protein around the food vesicle of Plasmodium resulted in a decrease in haemoglobin endocytosis and digestion of the parasite. This reduced the activation of artemisinin and made the parasite resistant to artemisinin. Therefore, we hypothesized that the differential expression of EtPGK, a key enzyme in the glycolysis pathway, in drug-resistant strains may be closely related to the generation of drug resistance of E. tenella.
qRT-PCR and western blot showed that compared with the DS strain of E. tenella, the transcription and translation levels of EtPGK were downregulated in the 2 drug-resistant strains and were negatively correlated with the drug concentrations of diclazuril and maduramicin. Enzyme activity assays showed that EtPGK activity was significantly downregulated in the DZR strain compared with the DS strain. Gene mutations may cause changes in expression level or enzyme activity. Our laboratory has previously performed whole-genome sequencing of DS strains and different diclazuril-resistant strains, and screened for differential SNPs of diclazuril-resistant strains compared to DS strains (data not published). However, we found that EtPGK was not screened for gene mutations in the DZR strains. The downregulation of EtPGK enzyme activity in the DZR strain may be caused by decreased expression level and other reasons, which requires further research. Western blot revealed that the protein level of EtPGK was significantly reduced in the MRR strain. Maduramicin kills parasites mainly by interrupting their normal intracellular ion balance and influencing Na+/K+-ATPase activity (Chapman et al., Reference Chapman, Jeffers and Williams2010). This may lead to changes in the content of metal ions such as Na+ and K+ in the MRR strain, thereby affecting enzyme activity. Lou et al. (Reference Lou, Cao, Duan, Tan, Li, Zhou and Wang2024) found that both K+ and Na+ have a significant effect on the activity of synthetic 7α-hydroxysteroid dehydrogenase. In addition, studies have shown that K+, Na+, Ca2+ and Mg2+ can promote the activity of esterolytic enzymes in cow rumen (Lee et al., Reference Lee, Cho, Ahmad, Patel, Kim, Jung, Jeong, Haque and Cho2021). We speculated that Mg2+ in the enzyme activity assay kit reaction solution might also have affected EtPGK activity. Compared with the DS strain, the mRNA transcription level of EtPGK in diclazuril-resistant strains isolated from the field was significantly downregulated. However, there was still a gap between the DZR strain that was induced in the laboratory and completely resistant to 1.2 ppm diclazuril. This may be because although field DZR strains have drug resistance, they are not completely resistant. Differences in their sensitivity to drugs also lead to differences in EtPGK expression levels. The study on Leishmania tropica found PGK to be differentially expressed in antimony-resistant field isolates (Kazemi-Rad et al., Reference Kazemi-Rad, Mohebali, Khadem-Erfan, Saffari, Raoofian, Hajjaran, Hadighi, Khamesipour, Rezaie, Abedkhojasteh and Heidari2013). PGK was found to be downregulated in cisplatin-resistant cell lines of oral squamous cell carcinoma, and a study of kidney renal clear cell carcinoma found that PGK enhanced sorafenib resistance and tumorigenesis by regulating glycolysis, which is a promising drug target (Nakatani et al., Reference Nakatani, Nakamura, Uzawa, Wada, Seki, Tanzawa and Fujita2005; He et al., Reference He, Luo, Huang, Zhang, Hou, Liang, Deng, Zhang and Liang2024).
Previous studies found that the upregulated expression of key enzymes of the glycolytic pathway in drug-resistant strains may provide energy for the parasite to antagonize drugs. However, our study found that EtPGK was downregulated in drug-resistant strains, which is interesting. Studies have found that the upregulation of mitochondrial PGK by the ROS-TBC1D15 pathway promoted neuronal death after oxygen-glucose deprivation/reoxygenation (OGD/R) injury, while knocking down PGK led to a reduction in OGD/R-induced neuronal death (Chen et al., Reference Chen, Wang, Chen, Cheng, Gao, Chen, Yao, Sun, Ren, Li, Che and Wan2024). Other studies showed that overexpression of PGK in H157 lung squamous cell carcinoma cells destroyed the stability of uPAR mRNA and reduced cell surface uPAR expression and uPAR-mediated signalling, mitosis, cell adhesion and migration, and other cellular functions (Shetty et al., Reference Shetty, Velusamy, Bhandary, Liu and Shetty2010). Liu et al. (Reference Liu, Shen, Sun, Wu and Xu2023) found that PGK aggravated cerebral ischaemia-reperfusion injury (CIRI) by inhibiting the Nrf2/ARE signalling pathway and that inhibiting PGK attenuated CIRI by activating the Nrf2/ARE pathway to reduce the release of inflammatory and oxidative factors in astrocytes. Therefore, we speculated that EtPGK might antagonize the effects of drugs on E. tenella by participating in other pathways, such as downregulating inflammatory pathways, inducing antioxidant pathways, or affecting the expression of parasite surface proteins. However, the specific mechanism of the relationship between EtPGK and the development of drug resistance in E. tenella requires further studies.
In addition to participating in the glycolytic pathway to provide energy for parasite activities, EtPGK may help E. tenella adapt to environmental changes,participate in parasites invasion of host cells and development of drug resistance. Compared with DS strain, the downregulated expression of EtPGK in resistant strains of E. tenella is different from the previous studies of some resistance-related genes. The reasons will be worth exploring in the future. With the development of gene editing in Eimeria, we will verify whether EtPGK is involved in the development of E. tenella drug resistance by overexpression of EtPGK in resistant strains or knockdown of EtPGK in DS strains. It will not only clarify the relationship between EtPGK and the emergence of drug resistance, but also help us to further understand the biological characteristics of EtPGK in E. tenella.
Conclusions
In this preliminary study on the function and characterization of EtPGK, we found that EtPGK was differentially expressed at different developmental stages of E. tenella, with the highest expression level at the SO stage. EtPGK was located on the surface of the parasites in addition to the cytoplasm of E. tenella. Compared with the DS strain, the expression levels of EtPGK in the DZR and MRR strains were significantly downregulated. The transcription levels of EtPGK were also significantly lower in the field DZR and SMR strains than in the DS strain. The enzymatic activity of EtPGK was lower in the DZR strain than in the DS strain. These results suggest that EtPGK may play multiple roles in addition to glycolysis. Moreover, EtPGK might be involved in the development of drug resistance of E. tenella to anti-coccidial drugs.
Data availability statement
All relevant data are within the paper. Sequence data are available on the NCBI GenBank database.
Author contributions
Y. Y. designed experiment, prepared test samples, performed the experiments and wrote the original draft. W. H. participated in the design and performed the experiment. Q. Z. and S. Z. participated in the collection of experimental samples. H. D. assisted with the experiment and analysed data. H. H. designed the experiments and revised the manuscript. All authors read, provided feedback and approved the final manuscript.
Financial support
This work was supported by the National Key R&D Program of China (2023YFD1802400), the National Natural Science Foundation of China (grant Nos. 31970420) and the National Sharing Service Platform for Parasite Resources (No. TDRC-2019-194-30). We thank all organizations that funded this work and all those who provided technical assistance.
Competing interests
None.
Ethical standards
All animal procedures were approved by the Animal Ethics Committee of Shanghai Veterinary Institute, Chinese Academy of Agricultural Science. Experiments were conducted in accordance with animal ethics guidelines and approved protocols.