In China, approximately 12 % of adult males are considered to be obese( Reference Xi, Liang and He 1 , Reference Li, Jiang and Hu 2 ). Since vitamin D nutritional status may be important in regulating obesity( Reference Wood 3 – Reference Vinh Quoc Lu’o’ng and Nguyen 6 ), interest was aroused to study Chinese obese males in this regard. Circulating 25-hydroxyvitamin D (25(OH)D; calcidiol), a metabolite of vitamin D having a half-life of 2–3 weeks, is recommended as a reliable marker of vitamin D nutritional status( Reference Jones 7 – Reference Holick, Binkley and Bischoff-Ferrari 10 ). The US Institute of Medicine( 11 ) reports that a circulating 25(OH)D concentration equal to 50 nmol/l (20 ng/ml) meets the vitamin D requirements of 97·5 % of the population, and this threshold is widely accepted for hypovitaminosis D( Reference Holick, Binkley and Bischoff-Ferrari 10 , Reference Malabanan, Veronikis and Holick 12 – Reference Forrest and Stuhldreher 14 ). Not satisfied with that basic requirement, many researchers claim that improvement of circulating 25(OH)D concentrations to much higher than 50 nmol/l can prevent a broad range of diseases( Reference Foss 4 , Reference Holick 15 – Reference Mithal, Wahl and Bonjour 17 ). In addition, 25(OH)D≤25 nmol/l (10 ng/ml) is considered to be ‘severe vitamin D deficiency’, 75–250 nmol/l (30–100 ng/ml) a ‘sufficient or optimal’ level and 50–75 nmol/l (21–29 ng/ml) an ‘insufficient’ level( Reference Malabanan, Veronikis and Holick 12 , Reference Kendrick, Targher and Smits 13 , Reference Holick 18 ), although these reference ranges are not defined by the Institute of Medicine( 11 ). Obesity is usually accompanied with low plasma or serum concentrations of vitamin D and its metabolite, 25(OH)D, as they are fat-soluble and most likely sequestered into the fat cells( Reference Liel, Ulmer and Shary 19 – Reference Blum, Dolnikowski and Seyoum 23 ).
Low vitamin D nutritional status is associated with two common phenomena in obesity: hypersecretion of parathyroid hormone (PTH) and insulin resistance (IR). IR is a key pathological change that causes many obesity complications( Reference Zeyda and Stulnig 24 , Reference Codogno and Meijer 25 ). The potential mechanism of vitamin D to improve insulin metabolism has been investigated( Reference Alvarez and Ashraf 26 ) and it has been postulated that improving vitamin D status in obese subjects can reduce IR and prevent chronic diseases( Reference Chiu, Chu and Go 27 ). However, confounding factors such as race( Reference Alvarez and Ashraf 26 ), body size( Reference Muscogiuri, Sorice and Prioletta 28 ) and others( Reference Manco, Calvani and Nanni 29 ) mean that identifying an association between vitamin D and elevated IR pathology is difficult. The relationship between vitamin D deficiency and high serum PTH is more clearly defined( Reference Holick 15 , Reference Kumar and Thompson 30 ). Under conditions of deficient 25(OH)D supply and decreasing tendency of 1,25-dihydroxyvitamin D (1,25(OH)2D; calcitriol) production, decreased PTH synthesis up-regulates 25(OH)D-1-α-hydroxylase to stabilize the circulating and intercellular 1,25(OH)2D concentrations( Reference Kumar and Thompson 30 , Reference Lips 31 ). Aside from regulating Ca homeostasis, 1,25(OH)2D has multiple roles after binding with the vitamin D receptor (VDR) in target tissues. During adipocyte differentiation, VDR mRNA level changes( Reference Burton, Guan and Nagarajan 32 , Reference Gerhold, Liu and Jiang 33 ) and plays a key role in mediating the actions of 1,25(OH)2D( Reference Kong and Li 34 ). The SNP of the VDR gene may affect its own transcription( Reference Arai, Miyamoto and Yoshida 35 ) or protein activity( Reference Arai, Miyamoto and Taketani 36 ). Interestingly, SNP of VDR are associated with numerous diseases( Reference Uitterlinden, Fang and Van Meurs 37 , Reference Valdivielso and Fernandez 38 ), including obesity( Reference Ochs-Balcom, Chennamaneni and Millen 39 , Reference Binh, Nakahori and Hien 40 ).
Owing to differences in living circumstances, dietary patterns and genetic background, the vitamin D status, VDR SNP profiles and the effects of oral vitamin D supplementation in obese subjects are not well known. We therefore examined the effects of these factors on vitamin D, PTH and IR status in a population of obese Chinese males in the city of Shenzhen (latitude 22·5°N).
Experimental methods
Cross-sectional survey
The ‘Shenzhen Survey for Chronic Diseases and Related Risk Factors 2009’ was conducted from 1 June to 31 July of 2009, using a stratified, multistage, cluster sampling method. This survey involved 3746 adult males (age 18–69 years) who had lived in Shenzhen, China for ≥5 years( Reference Lei, Zhou and Xiong 41 ). From this epidemiological survey, individuals meeting the following criteria were included for the comparative analysis on obese and normal-weight males: (i) an obese group, with BMI ≥28·0 kg/m2 ( Reference Chen and Lu 42 ); (ii) a normal-weight group, with 18·5 kg/m2≤BMI<24·0 kg/m2 ( Reference Chen and Lu 42 ), and normal values for blood pressure, fasting plasma glucose, lipids, uric acid and creatinine being apparent; and (iii) all were Han Chinese and did not smoke, drink nor take vitamin D supplements in the past >2 years.
Oral vitamin D supplementation trial protocol
From June to August of 2011, a human intervention trial was conducted with volunteers from the above cross-sectional survey who were living in the community where the Shenzhen Center for Chronic Disease Control is located. The trial was approved by the Ethics Committee of the Center. The trial number registered on the ClinicalTrials.gov site is NCT01781169. After signing the informed consent form, the accepted adult Chinese males were grouped into an obese group (twenty-two participants) and a normal-weight group (twenty-one participants). The sample sizes for both groups also met those calculated based on their circulating 25(OH)D concentrations with 95 % confidence interval and an expected sampling error of 5 nmol/l. In addition to the inclusion criteria in the case–control study, the following criteria were also met for both groups: (i) non-vegetarian; (ii) fasting serum glucose<7·0 mmol/l; (iii) serum glucose 2 h after 75 g oral glucose loading<11·1 mmol/l; and (iv) normal functioning of liver and kidney evaluated by alkaline phosphatase, aspartate aminotransferase, creatinine and uric acid. Information about intake of foods containing vitamin D in the past year, health status and other lifestyle factors in the past 5 years was collected by face-to-face interview with a set of questionnaires. Food replicas (Zhending Computer Technology Co., Ltd, Shanghai, China) were used to facilitate the recall of food intakes. Vitamin D intakes were estimated according to the participants’ food intake and the reference vitamin D contents of foods published by the US Department of Agriculture( 43 ). Anthropometric parameters including height, body weight, skinfold thickness, waist circumference and hip circumference were measured according to standard protocols( Reference Lohman, Roche and Martorell 44 ).
With the baseline information and blood samples of fasting state and oral glucose tolerance testing collected in late June, all participants received the oral vitamin D intervention starting from the same day in the catering division of the Shenzhen Center for Chronic Disease Control. Based on previous supplement methods( Reference Malabanan, Veronikis and Holick 12 , Reference Barger-Lux, Heaney and Dowell 45 – Reference Heaney, Recker and Grote 47 ), every participant of the two groups consumed an oral capsule containing 50 000 IU (1250 μg) of natural vitamin D3 (catalogue no. 7184–01; Bio-Tech Pharmacal, Inc., Fayetteville, AR, USA) with a meal once weekly for 8 weeks, under the observation of project staff. Any participant who would be inevitably absent from the observation of vitamin D intake informed the project staff in advance to get the vitamin D capsule and reported the scheduled intake via telephone. During the vitamin D supplementation period, the participants were required to maintain their normal living and eating habits. Every other day, a telephone interview was conducted to know their duration of sun exposure, dress and usage of sunscreen and sunshade between 10.00 and 15.00 hours when exposure to UV light causes the most effective vitamin D synthesis in skin( Reference Holick 48 ), as well as to monitor symptoms of possible vitamin D toxicity, such as anorexia, nausea, vomiting, diarrhoea, constipation, continuous headaches, irregular heartbeat, muscle and bone pain, etc.( Reference Alshahrani and Aljohani 49 ). These symptoms were also cared for by an endocrine physician when participants came to the catering division to take vitamin D supplements every week and clinical examinations would be done if necessary. One week after the last intake of vitamin D supplement, all biometric and biochemical measures were re-evaluated. The vitamin D intake in the past 8 weeks was surveyed again by face-to-face interview with the FFQ and food replicas.
Sample collection and preparation
In the cross-sectional study, overnight fasting (>10 h) blood samples were drawn from the ulnar vein and collected in sodium fluoride/potassium oxalate tubes. Within 2 h, the plasma was separated for subsequent chemical analyses and blood cells were collected for DNA extraction. In the intervention trial, fasting blood was collected in a vacuum tube without anticoagulant for serum separation and a K-EDTA tube for whole blood parameter assays. Samples were used for some instant determinations or stored at −75°C for later use.
Biochemical analysis
Serum insulin was analysed with a chemiluminescence kit (catalogue no. 33410; Beckman Coulter Inc., Guangzhou, China) in an automatic immunoassay analyser (ACCESS2; Beckman Coulter Inc.). Glucose, TAG, total cholesterol, HDL cholesterol, LDL cholesterol, uric acid, creatinine, alkaline phosphatase and aspartate aminotransferase were analysed with an automatic biochemical analyser (AU400; Olympus Co., Ltd, Beijing, China). The IR status was evaluated with the homeostasis model assessment of insulin resistance index (HOMA-IR)( Reference Matthews, Hosker and Rudenski 50 ). The calculation was: HOMA-IR=fasting serum glucose (mmol/l)×fasting serum insulin (mIU/l)/22·5. Whole-blood Ca was determined with atomic absorption spectrometry (BH5100; Bohui Innovation Technology Co., Ltd, Beijing, China).
Using a microplate reader (Multiskan FC; Thermo Fisher Scientific Inc., Beijing, China), the 25(OH)D concentrations of all plasma and serum samples were measured by enzyme immunoassay (catalogue no. AC-57F1; IDS Ltd, Tyne & Wear, UK). This method met the performance target set by the Advisory Panel of the Vitamin D External Quality Assessment Scheme. The intact PTH (iPTH) concentration of the fasting serum was also determined by enzyme immunoassay (catalogue no. 7022; Biomerica Inc., Irvine, CA, USA). The intra- and inter-assay CV for the 25(OH)D assay were <8 %, and for the iPTH assay were <7 %.
Genotyping of SNP
Five SNP, ApaI, TaqI, FokI, rs3782905 and Cdx-2, of the VDR were analysed using a PCR–restriction fragment length polymorphism method. Briefly, total DNA was extracted from approximately 200 μl of anticoagulated whole blood with a DNA extraction kit (QIAamp DNA mini kit, catalogue no. 51106; QIAGEN Co., Ltd, Shanghai, China). Four pairs of primers were designed to amplify the four DNA fragments containing the five SNP, respectively (see online supplementary material, Supplemental Table 1). Approximately 1 μg of the purified PCR product was then incubated with the corresponding restriction enzyme for ApaI, TaqI, FokI (catalogue no. D1005A, D1189A and D1046A, respectively; TaKaRa Biotech Co., Ltd, Dalian, China), DdeI (catalogue no. R6295; Promega Biotech Co., Ltd, Beijing, China) or BseMII (catalogue no. ER1401; Thermo Fisher Scientific Co., Ltd., Beijing, China) in a volume of 20 μl. Finally, the enzyme-digested product was loaded into a 2 % (w/v) agarose gel (BioWest Regular Agaros G-10, catalogue no. 111860; Gene Co. Ltd, Hong Kong, China) for electrophoresis and determination of genotypes.
Statistical analysis
To compare the normal-weight and obese groups in the cross-sectional survey or the intervention trial, parametric variables were analysed with a univariate general linear model using age as a covariate. Based on the obesity prevalence in adult Shenzhen males at different ages( Reference Lei, Zhou and Xiong 41 ) and the age composition in the present study, age was divided into three periods: <35 years, 35–<50 years and ≥50 years. To examine the vitamin D status and SNP genotypes in the cross-sectional survey, the χ 2 method was applied to test the differences within each age period followed by Cochran’s and Mantel–Haenszel statistics controlling any effects of age. The SNP were analysed with various genetic models( Reference Lewis 51 ) including genotypic frequency, allelic frequency (multiplicative model), dominant model and recessive model. Partial correlation analysis was conducted between the plasma 25(OH)D concentrations and each of the SNP genotypes adjusting for group, age and BMI. The frequencies of each SNP in both groups were tested for Hardy–Weinberg equilibrium. Within the same group in the intervention trial, data were analysed with the paired-samples t test. The Mann–Whitney U test was used to analyse the data with non-normal or unknown distribution. Comparisons were considered statistically significant if P<0·05.
Results
Vitamin D status and SNP of VDR gene
The biometric and biochemical characteristics of the participants (eighty-two normal-weight and ninety-nine obese men) are summarized in Table 1. The majority of the indices examined were significantly worse for the obese group compared with the normal-weight group (P<0·01). Only the mean concentrations of plasma 25(OH)D (55·3 (sd 12·2) nmol/l in the normal-weight group and 54·8 (sd 13·4) nmol/l in the obese group, P=0·877) and creatinine (P=0·301) were not significantly different. None of the indicators were correlated with BMI, waist:hip ratio, blood pressure, plasma lipid, uric acid or creatinine (P>0·10). The calculated Ca intakes based on the FFQ in normal-weight and obese groups were 650·6 (sd 282·7) mg/d and 639·1 (sd 266·2) mg/d, respectively (Mann–Whitney U test, P=0·947). Also, the percentage of participants with plasma 25(OH)D concentration <50 nmol/l, 50–<75 nmol/l and 75–250 nmol/l in both groups (38·4 %, 51·5 %, 10·1 % for obese v. 29·3 %, 64·6 %, 6·1 % for normal weight) were not significantly different without (P=0·192) or with (P=0·749) adjustment for age (Fig. 1). Thirty to forty per cent of participants in both groups had a plasma 25(OH)D concentration <50 nmol/l, and none had a concentration <25 nmol/l or >250 nmol/l.

Fig. 1 Percentage distribution of circulating serum 25-hydroxyvitamin D concentrations in normal-weight (NW) and obese males (![]() , <50 nmol/l;
, <50 nmol/l; ![]() , 50–<75 nmol/l;
, 50–<75 nmol/l; ![]() , 75–250 nmol/l), Shenzhen City, Guangdong Province, China. The percentages of participants in the different categories of vitamin D status did not differ between the NW (n 82) and obese (n 99) groups, with or without adjustment for age (P>0·05)
, 75–250 nmol/l), Shenzhen City, Guangdong Province, China. The percentages of participants in the different categories of vitamin D status did not differ between the NW (n 82) and obese (n 99) groups, with or without adjustment for age (P>0·05)
Table 1 Biometric and biochemical data of the adult males for the case–control study, Shenzhen City, Guangdong Province, China

25(OH)D, 25-hydroxyvitamin D.
* The P value for age was obtained using the independent-samples t test; the P values of all other parameters were obtained by a univariate general linear model with age as a covariate.
The genotypic and allelic frequencies of the five VDR SNP are summarized in the online supplementary material, Supplemental Table 2. The P values of statistical analyses are listed in Table 2. Only the genotypic frequency of rs3782905 differed significantly between the two groups when age was considered as a covariate in the Cochran’s and Mantel–Haenszel statistics (P=0·043). Tendency to be different between the obese and the normal-weight groups (0·05<P<0·10) was found in the genotypic frequency and dominant model of ApaI in the age period of 35–<50 years, and in the allelic frequency and recessive model of Cdx-2 with age as a covariate in the Cochran’s and Mantel–Haenszel statistics. The remaining P values for comparisons of the five SNP in various genetic models were not significantly different between the two groups (P>0·10). No VDR genotypes based on the SNP had a correlation with plasma 25(OH)D when adjusted for age, BMI and experimental group (P>0·05, see online supplementary material, Supplemental Table 3).
Table 2 Statistical comparisons (P values) of five VDR SNP between the obese (n 99) and normal-weight (n 82) adult males, Shenzhen City, Guangdong Province, China, analysed with different genetic models
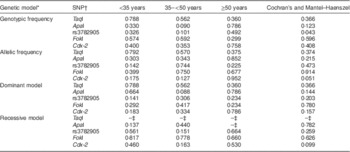
VDR, vitamin D receptor gene.
* The dominant model is used for major homozygotes v. combination of minor homo- and heterozygotes. The recessive model is used for combination of major homo- and heterozygotes v. minor homozygotes.
† RefSNP ID numbers are: rs731236 for TaqI, rs7975232 for ApaI, rs2228570 for FokI and rs11568820 for Cdx-2.
‡ The P value was not available due to at least one sum of rows or columns being zero.
Biometric profile and lifestyle factors in the intervention trial
Excluding those with significant changes of lifestyle and final BMI (>3 %), twenty-one participants of each group were accepted for data analyses. The biometric characteristics of the two groups were similar to those in the cross-sectional survey (see online supplementary material, Supplemental Table 4). The final BMI levels were 21·8 (sd 1·2) kg/m2 for the normal-weight group and 30·2 (sd 1·7) kg/m2 for the obese group, without significant differences compared with the respective values before vitamin D supplementation.
The dietary vitamin D intakes of the two groups in the past 1 year were not significantly different (Mann–Whitney U test, P=0·910; median 67·1 IU/d or 1·68 μg/d). During the intervention trial, all participants maintained their normal dietary habits and data collected by the FFQ showed they did not increase their intake of vitamin D-rich foods. The duration of sun exposure with summer clothing (usually T-shirt and trousers) from 10.00 to 15.00 hours was not significantly different (Mann–Whitney U test, P=0·339; median sun exposure 19·8 min/d). In addition, there were no complaints of feeling uncomfortable or of symptoms potentially caused by vitamin D toxicity( Reference Alshahrani and Aljohani 49 ).
Changes in biochemical indices by vitamin D supplementation
The changes of IR and serum concentrations of 25(OH)D, iPTH and insulin of both normal-weight and obese groups, before and after the intervention trial, are summarized in Fig. 2. The average baseline serum 25(OH)D concentrations were 52·8 (sd 17·8) nmol/l for the normal-weight group and 46·1 (sd 9·1) nmol/l for the obese group, and this difference was not significant (P=0·071). Findings were similar to the cross-sectional survey. After vitamin D supplementation, the serum 25(OH)D concentration of both groups was enhanced significantly (P<0·001), with a 2·8-fold increase for the normal-weight group and a 1·6-fold increase for the obese group. Thus the normal-weight group had a significantly higher concentration (P<0·001) of serum 25(OH)D (181·3 (sd 30·2) nmol/l) than the obese group (116·7 (sd 20·3) nmol/l) after the intervention trial. All participants in the normal-weight group had serum 25(OH)D concentration ≥75 nmol/l, while 9·5 % of the obese participants still had this index between 50 and <75 nmol/l.

Fig. 2 Changes in fasting serum concentrations of (a) 25-hydroxyvitamin D (25(OH)D), (b) intact parathyroid hormone (iPTH), (c) insulin (FSI) and (d) homeostasis model assessment of insulin resistance index (HOMA-IR), before (![]() ) and after (
) and after (![]() ) the vitamin D supplementation trial in normal-weight (NW) and obese males, Shenzhen City, Guangdong Province, China. Data are means with their standard errors represented by vertical bars (twenty-one participants per group). a,b,cWithin the same group, or within the same stage between groups using age as a covariate, data with unlike superscript letters were significantly different (P<0·05). With age adjusted, before and after the trial, P values for 25(OH)D were 0·071 and <0·001, for iPTH were 0·023 and 0·034, for FSI were <0·001 and 0·010, and for HOMA-IR were <0·001 and 0·023, respectively. Without adjustment, before and after the trial, P values for 25(OH)D were 0·134 and <0·001, for iPTH were 0·038 and 0·030, for FSI were <0·001 and 0·003, and for HOMA-IR were <0·001 and 0·003, respectively
) the vitamin D supplementation trial in normal-weight (NW) and obese males, Shenzhen City, Guangdong Province, China. Data are means with their standard errors represented by vertical bars (twenty-one participants per group). a,b,cWithin the same group, or within the same stage between groups using age as a covariate, data with unlike superscript letters were significantly different (P<0·05). With age adjusted, before and after the trial, P values for 25(OH)D were 0·071 and <0·001, for iPTH were 0·023 and 0·034, for FSI were <0·001 and 0·010, and for HOMA-IR were <0·001 and 0·023, respectively. Without adjustment, before and after the trial, P values for 25(OH)D were 0·134 and <0·001, for iPTH were 0·038 and 0·030, for FSI were <0·001 and 0·003, and for HOMA-IR were <0·001 and 0·003, respectively
Either before or after vitamin D supplementation, with age adjusted, iPTH (P=0·023 and 0·034, respectively), fasting serum insulin (P=<0·001 and 0·010, respectively) and HOMA-IR (P=<0·001 and 0·023, respectively) in the obese group were higher (P<0·05) than those in the normal-weight group. However, the fasting serum glucose values did not differ significantly between them (P=0·465 and 0·643, respectively). Before the trial, the normal-weight group had a lower glucose concentration 2 h after the oral glucose challenge test compared with the obese group (data not shown). After vitamin D supplementation, iPTH, fasting serum insulin and HOMA-IR did not change significantly (P>0·05) in the normal-weight group, but decreased significantly in the obese group (P<0·05).
Within each group, lipid profile, uric acid, blood Ca, creatinine, alkaline phosphatase and aspartate aminotransferase did not significantly change (P>0·05) after vitamin D supplementation (see online supplementary material, Supplemental Table 5). However, in the respective normal reference ranges, HDL cholesterol decreased (P=0·036) and aspartate aminotransferase increased (P=0·032) in the obese group, and uric acid increased (P=0·014) in the normal-weight group.
Discussion
Among the risk factors associated with the growing incidence of various chronic diseases in the Chinese population, including obesity, vitamin D nutritional status has been gaining attention. Only a small number of studies have reported the high prevalence of hypovitaminosis D among the Chinese population. A dual-centre study demonstrated that more than 90 % of young women in both Hong Kong and Beijing had 25(OH)D concentrations ≤50 nmol/l in spring, and 18 % of young women in Hong Kong had 25(OH)D concentrations ≤25 nmol/l( Reference Woo, Lam and Leung 52 ). A survey, conducted in Beijing (~40°N) and Shanghai (~31°N) from April to June in 2005, revealed that 69 % of the middle-aged and elderly people were vitamin D ‘deficient’ (25(OH)D<50 nmol/l) and 24 % were vitamin D ‘insufficient’ (50 nmol/l≤25(OH)D<75 nmol/l)( Reference Lu, Yu and Pan 53 ). Furthermore, blood samples collected during April and May from healthy pregnant women and their newborn infants in a Beijing hospital indicated that none of the subjects had serum 25(OH)D concentration >75 nmol/l and half of them were in ‘severe vitamin D deficiency’ (25(OH)D<25 nmol/l)( Reference Song, Si and Liu 54 ).
In the present study, approximately a third of adult males in Shenzhen, a southern Chinese city located at 22·5°N, had circulating 25(OH)D concentration <50 nmol/l, and approximately 90 % had a concentration <75 nmol/l (even during summer). Although the study size was relatively small, participant selection adhered to strict inclusion criteria to limit potential confounders and generate reliable data. These low vitamin D statuses may arise from people limiting their sun exposure to avoid hot weather and sunburn despite sunny conditions in the low-altitude regions. Besides, variable responsiveness to sun exposure is another newly assumed mechanism underlying the low vitamin D status( Reference Binkley, Novotny and Krueger 55 ), which needs further investigation in this Chinese population.
Obese individuals have a lower circulating 25(OH)D concentration than those of normal weight( Reference Liel, Ulmer and Shary 19 – Reference Blum, Dolnikowski and Seyoum 23 ). However, in adult males living in subtropical China, we did not find a significantly lower vitamin D status in the obese group. The following factors should be considered when interpreting this apparent inconsistency. First, the 25-hydroxylase enzyme that catalyses hydroxylation of vitamin D into 25(OH)D may not have been saturated or the fat storage effect was weak at the present baseline vitamin D intake of <50 μg/d (2000 IU/d) and serum 25(OH)D concentration of <100 nmol/l, as previously reported( Reference Heaney, Armas and Shary 56 ). However, with increasing intake or supplementation of vitamin D, the fat storage effect and the decreased bioavailability of vitamin D in the obese participants( Reference Wortsman, Matsuoka and Chen 20 ) were revealed. Second, the mean BMI of our obese participants was lower than those in Western population studies; the BMI range for obesity is ≥28·0 kg/m2 in China but ≥30·0 kg/m2 in Western countries( Reference Bei-Fan 57 ). This is because Chinese populations are more likely to develop negative health consequences at a lower BMI than Caucasians. Third, other reasons associated with genetic makeup, lifestyle and environmental differences among various populations may contribute to the differences observed.
The bioactive form of vitamin D, 1,25(OH)2D, is actually a hormone with its serum concentration strictly controlled by sophisticated mechanisms. Serum 1,25(OH)2D is resistant to a broad range of vitamin D (25, 250 or 1250 μg/d for 8 weeks) or 25(OH)D (10, 20 or 50 μg/d for 4 weeks) intakes( Reference Barger-Lux, Heaney and Dowell 45 ). It can enter many types of cell to form a complex with the VDR, modulates the expressions of more than 200 genes via vitamin D response elements (VDRE) and performs various biological functions( Reference Ramagopalan, Heger and Berlanga 58 ). VDR is important in the function of 1,25(OH)2D, and itself is a 1,25(OH)2D-targeted gene with VDRE( Reference Maestro, Davila and Carranza 59 ). Multiple studies have been performed to explore the relationship between VDR gene SNP and VDR-mediated biological activities or diseases, and to understand the mechanisms( Reference Uitterlinden, Fang and Van Meurs 37 , Reference Valdivielso and Fernandez 38 ). Cdx-2 polymorphism is located in the promoter region and thus its variant has the possibility to impact on VDR transcription( Reference Arai, Miyamoto and Yoshida 35 ). FokI variation occurs in the second exon of VDR and its polymorphism results in two different lengths and activities of VDR peptides( Reference Arai, Miyamoto and Taketani 36 , Reference Uitterlinden, Fang and Van Meurs 37 ). Near the 3′-end of VDR, BsmI and ApaI are located in the intron, between exons 8 and 9, and TaqI is in exon 9( Reference Uitterlinden, Fang and Van Meurs 37 ). These three polymorphisms have a strong linkage disequilibrium extending into the 3′-regulatory region containing the untranslated region. The 3′-untranslated region of a gene is usually involved in its own expression regulation and, therefore, BsmI, ApaI and TaqI were reported to have associations with a variety of diseases( Reference Valdivielso and Fernandez 38 ). However, there are many SNP in the intron region of VDR with unknown biological functions. The rs3782905 SNP (between exons 2 and 3 with unknown function) in females aged 35–80 years (n 1773)( Reference Ochs-Balcom, Chennamaneni and Millen 39 ) and ApaI SNP in postmenopausal women (n 140)( Reference Binh, Nakahori and Hien 40 ) are associated with obesity, whereby BMI was not defined as clearly as the two groups in our study. In our cross-sectional survey involving 181 adult males, the significance (P=0·043) of rs3782905 was demonstrated, while ApaI had only difference tendency (P=0·090) in those aged 35–<50 years. Also, Cdx-2 tended to be different in our findings based on allelic frequency (P=0·051) and the recessive model (P=0·099). The consistency and inconsistency of our findings compared with previous studies( Reference Ochs-Balcom, Chennamaneni and Millen 39 , Reference Binh, Nakahori and Hien 40 ) might be partly explained by our BMI grouping methods as well as the genetic variation of VDR SNP in different populations( Reference Lins, Vieira and Grattapaglia 60 ).
PTH is another target gene of 1,25(OH)2D with a negative VDRE on its promoter( Reference Kim, Fujiki and Murayama 61 ). Transactivation of PTH is sensitive to decreases in 1,25(OH)2D in order to tightly maintain its constant level. However, prolonged high levels of PTH can impair intestinal Ca absorption and stimulates osteoclasts to dissolve the mineralized collagen matrix in bone, causing many bone diseases( Reference Holick 15 ). Holick and co-workers( Reference Malabanan, Veronikis and Holick 12 ) demonstrated the significant reduction of PTH and its inverse association with serum 25(OH)D when adults with an initial 25(OH)D concentration below 50 nmol/l received vitamin D supplementation. Recently, the circulating PTH level was reported to become stable at serum 25(OH)D level of 46·2 nmol/l, as estimated by the segmented model( Reference Saliba, Barnett and Rennert 62 ). In obese individuals with low vitamin D status, secondary hyperparathyroidism and elevated serum PTH levels are also evident( Reference Bell, Epstein and Greene 63 – Reference Snijder, van Dam and Visser 65 ). Steingrimsdottir et al.( Reference Steingrimsdottir, Gunnarsson and Indridason 66 ) found that at a low serum 25(OH)D concentration of <18 ng/ml (45 nmol/l), Ca intake of <800 mg/d (compared with >1200 mg/d) was significantly associated with a high serum PTH. The Ca intakes of the two groups in our intervention trial were likely to be <800 mg/d, like those of the groups in the cross-sectional survey, and based on their recorded dietary patterns Ca intake probably did not change throughout the trial. More importantly, their baseline circulating 25(OH)D concentration was >18 ng/ml and there was no difference between groups. Thus the Ca intakes probably had little or no contribution to the changes of serum iPTH concentrations after the vitamin D supplementation trial. Therefore, the significant reduction in serum PTH concentration in the obese group (from 61·5 to 51·1 pg/ml, P=0·037) and the decline in the normal-weight group (from 47·1 to 38·3 pg/ml, P=0·058) were likely to be attributed to the significantly improved serum 25(OH)D concentrations.
Although cross-sectional analyses and laboratory experiments suggest that vitamin D deficiency and obesity are associated with the weakened response of cells to insulin, predisposed IR and pancreatic β-cell dysfunction( Reference Alvarez and Ashraf 26 , Reference Chiu, Chu and Go 27 , Reference Riachy, Vandewalle and Moerman 67 – Reference Kabadi, Lee and Liu 69 ), intervention outcomes were not always consistent. Variations may be due to differences in study populations and intervention protocols. Using obese Chinese males as subjects, we tested the benefit of large-dose vitamin D supplementation at 50 000 IU (1250 μg) once weekly for 8 weeks, similar to the protocol applied previously( Reference Malabanan, Veronikis and Holick 12 , Reference Barger-Lux, Heaney and Dowell 45 , Reference Heaney, Recker and Grote 47 ); this is a recommended strategy to treat adults who are vitamin D ‘deficient’( Reference Holick, Binkley and Bischoff-Ferrari 10 ). As a result, the IR status and insulin sensitivity in this population were significantly improved, consistent with the recent findings from India and New Zealand( Reference Nagpal, Pande and Bhartia 70 , Reference von Hurst, Stonehouse and Coad 71 ).
Conclusions
In summary, baseline vitamin D nutritional status did not differ between obese and normal-weight men living in subtropical China in summer. However, oral high-dose vitamin D supplementation revealed decreased vitamin D bioavailability in obese men and reduced their hypersecretion of PTH and insulin resistance. In addition, the rs3782905 SNP of the VDR gene was associated with obesity. The SNP of Cdx-2 and ApaI are candidates for future study in the relationship between VDR SNP and obesity, especially in a Chinese population. Finally, the generally held viewpoint in Chinese populations that high-dose administration of fat-soluble vitamin D has negative side-effects was a significant obstacle to recruiting a larger number of volunteers and implementing a longer trial period. Consequently a placebo control group or a placebo experimental stage was not established in the present study. Large-scale human trials avoiding these limitations are required to corroborate the conclusions and implications of our study.
Acknowledgements
Acknowledgements: The authors thank Ms Ya-Juan Song, Ms Willa Dong and Mr Shi-Jie Zheng for their help in the intervention trial, manuscript preparation and data analysis, respectively. Financial support: This project was funded by grants from the Danone Institute; China Diet Nutrition Research & Communication (grant number DIC2010-03); Science & Technology Innovation Commission of Shenzhen Municipality (grant number JCYJ20130402154801097); and the National Key Technology Research and Development Program of China (grant number 2012BAI02B02). None of these agencies had any role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: J.-C.Z. was responsible for the study design, data analysis and manuscript preparation. Y.-M.Z., Z.C., J.-L.M. and J.W. contributed to sample preparation and management and biomarker assays. F.-Z.X. and Y.-H.W. conducted blood sampling. P.G., J.P., J.X. and X.-L.L. coordinated the departments involved in the study and provided general assistance with the study. All authors have read and approved the final manuscript. Ethics of human subject participation: The trial was approved by the Ethics Committee of the Shenzhen Center for Chronic Disease Control (trial registration number on ClinicalTrials.gov site: NCT01781169).
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1368980014002845







