INTRODUCTION
Norovirus is the most common known cause of infectious intestinal disease in Western Europe and North America [Reference de Wit1–Reference Fretz3] and one of the leading causes of foodborne outbreaks of acute gastroenteritis [Reference Green, Knipe and Howley4–Reference Widdowson, Monroe and Glass9]. It has been estimated that there are over 600 000 cases of norovirus infection in England each year [Reference Wheeler10], with infection rates peaking during the winter months. Norovirus is highly infectious and can be transmitted in a variety of ways including contact with infectious individuals, contact with contamination in the environment and consumption of contaminated food. It is estimated that over 10% of norovirus cases in England and Wales are foodborne [Reference Adak11].
On 25 February 2009 the Health Protection Agency (HPA) was notified of four individuals who had developed symptoms of diarrhoea and vomiting within 48 h of having a meal at a restaurant in South East England. The HPA contacted the appropriate local authority (LA) which has statutory enforcement powers in food-borne outbreaks. They had received a report late the previous evening (24 February 2009) from private consultants contracted by the restaurant. Initial investigations revealed that the restaurant's management had employed the private consultants in mid-February to review its food safety management system following complaints of illness from diners at the restaurant. The complaints continued and the restaurant subsequently closed voluntarily on the 22 February 2009. The restaurant made no contact with either the LA or HPA prior to the 24 February 2009. Sixty-six complaints of illness had been received by the restaurant by the time it had contacted the LA, no complaints had been reported directly to the LA by diners.
This restaurant serves approximately 1750 customers per month. The restaurant uses an approach based on the principles of ‘molecular gastronomy’ [12, Reference Matthew13], prepares and serves unusual dishes using what it describes as innovative methods. The restaurant serves an à la carte menu and a ‘tasting menu’ which is composed of 16 different courses. The menu remains the same every day for both lunch and dinner. Approximately 95% of diners choose the tasting menu.
The restaurant employs over 60 staff, the majority of whom work full-time. These include chefs, kitchen staff, front of house staff, sommeliers, administrators and kitchen porters. The restaurant also runs chef and experimental kitchen stagiaire placement programmes.
It was recognized by the HPA that a large outbreak of possible foodborne illness had occurred at the restaurant. Descriptive analysis was performed to describe the size of the outbreak and the affected population, the nature of illness in diners, the pattern of illness over time and assess evidence for secondary spread.
A case-control study was performed to identify specific risk factors in order to make recommendations to assist in the prevention and control of future outbreaks.
METHODS
Epidemiological investigation
Cases were initially defined as anyone with a complaint of gastrointestinal illness who attended the restaurant from the time of the Christmas break re-opening on 6 January 2009 and its voluntary closure on 22 February 2009. The restaurant provided a contact list of individuals belonging to dining parties who reported illness after dining at the restaurant between these dates. A secure web-based structured questionnaire was developed and a link emailed to dining parties that included at least one symptomatic person to obtain demographic, clinical and risk factor information. They were also asked to report cases of gastrointestinal illness in their households during the same or the following week in order to obtain details about possible secondary cases. In total 223 emails were sent to complainants from 215 parties which comprised a total of 591 diners. The median party was n=2 (range 2–6).
The study population for the case-control study consisted of groups of diners who had eaten at the restaurant between 6 January 2009 and its closure on 22 February 2009 in which at least one member of the dining party they were part of had gastrointestinal symptoms. A case was defined as an individual reporting at least two symptoms of nausea, vomiting, and diarrhoea within 7 days of eating at the restaurant. Controls were defined as individuals from these groups with identified cases who had not displayed any of the above symptoms during the same time period.
A series of closed and open questions on menus and specific foods eaten at the restaurant were analysed using univariate and multivariable analysis. For the univariate analysis, where a dish was reported to have been eaten in part a value of 0·5 was given for consumption compared to 1 if eaten in full and 0 if not eaten. Multivariable analysis was performed for those exposures showing statistical evidence (at P<0·1) on univariate analysis with a stepwise exclusion approach using a cut-off of P<0·05. All analyses were performed using Stata 10.0 (Stata Corporation, USA).
Fifty-seven staff members were also interviewed by telephone. This included questions on their work, whether they had been ill and exclusion from working during and following illness.
Human microbiological investigation
Cases who had eaten at the restaurant in the week before closure were contacted to ascertain whether they still had gastrointestinal symptoms. Those that were symptomatic were asked to provide stool samples and these were tested for both bacteria and for the presence of norovirus by reverse-transcription–polymerase chain reaction (RT–PCR). In addition stool samples were obtained from 60/63 staff members, of which 44 were tested for norovirus; the remaining samples were not tested as the results were sufficient to confirm substantial norovirus infection in staff. Norovirus detection and strain characterization by genotyping were performed as described previously [Reference Kageyama14].
Food and environmental investigations
Environmental sampling using swabs was performed to detect contamination. This included sampling of all kitchen and food preparation areas as well as the dining area. A ‘deep cleaning’ exercise of the premises had been conducted immediately after closure and preceding notification of authorities, thus severely limiting the potential findings from environmental sampling as the environmental samples were taken 1 week after closure. The environmental samples were tested using national standards by a Regional HPA Food, Water and Environment (FWE) Laboratory.
In addition, food sampling was performed on a number of foods obtained from the restaurant. These samples were primarily frozen items such as stocks, sauces and purees. There was limited food to sample (no fresh products) as the restaurant had not prepared and served food since lunchtime on 22 February 2009, a period of more than 8 days. Samples were submitted to the Regional HPA FWE Laboratory.
Environmental health investigations included a detailed examination of menus, food items, suppliers, preparation methods, food hygiene practices and the food safety management system. Food storage facilities, preparation and kitchen facilities were reviewed. Members of staff were interviewed to determine food hygiene practice and their use of the facilities in their day-to-day work for the complex dishes which required substantial manipulation of food. As the restaurant was closed this was undertaken by interview rather than observation.
RESULTS
Epidemiology
Descriptive epidemiology
There were 591 people identified as belonging to dining parties where at least one person from the group had reported some symptoms of gastrointestinal illness and who had visited the restaurant during the outbreak period. Of these 591 diners, a completed questionnaire was received from 386; a response rate of 65%. Of the 386 responses, 240 met the outbreak case definition. An epidemic curve describing cases by date of onset of illness is shown on Figure 1. This showed an increasing number of illness reports in diners and staff from 6 January to 27 February 2009.
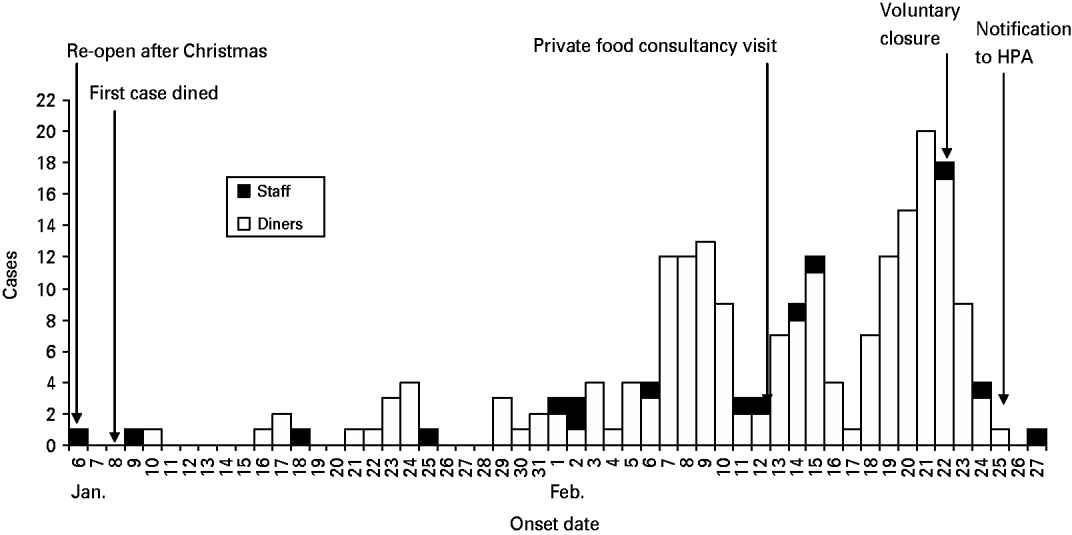
Fig. 1. Onset of illness in diners and staff at the restaurant, South East England, 6 January to 27 February 2009.
The median age was 38 years for cases and 36 years for controls. For both cases and controls 51% were female. The median time between eating and becoming unwell was 33 h, with a median reported duration of illness of 3 days. Of the 199 cases with a recorded date of onset of symptoms, 67% reported onset of illness between 24 h and 48 h after eating. The most frequently reported symptoms were diarrhoea (82%), nausea (78%), vomiting (73%) and abdominal pain (65%). There were no reports of any one requiring hospitalization as a result of illness. Six subsequent cases of similar illness were reported in household contacts who did not dine at the restaurant suggesting secondary person-to-person transmission. Faecal samples were not available for testing.
Analytical epidemiology
We performed a case-control study to assess evidence for association of illness with potential risk factors such as foods eaten at the restaurant and when diners ate at the restaurant. Of those that responded, 240 individuals met the case definition and 79 met the control definition. The remaining 67 respondents reported illness but did not meet the case definition symptoms or reported symptom onset more than 7 days after eating at the restaurant and were therefore excluded from the analysis.
Univariate analysis was performed for each dish. There was no evidence that the risk of illness varied between lunch or dinner but there was a increased risk in those who reported eating from the tasting menu compared to those eating the à la carte menu [odds ratio (OR) 2·7, 95% confidence interval (CI) 1·1–6·4] with 95% of cases reporting consumption from the tasting menu. Consumption of several food items from the tasting menu was individually associated with illness. The strongest associations on univariate analysis are shown in Table 1.
Table 1. Food items on the tasting menu associated with illness by univariate analysis
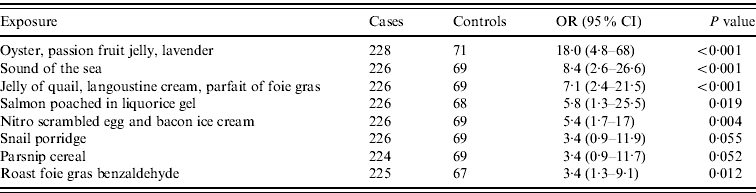
OR, Odds ratio; CI, confidence interval.
In the final multivariate model the only dish that remained statistically significant was the ‘oyster, passion fruit jelly, lavender’ dish (OR 7·0, 95% CI 1·1–45·2, P<0·040). Table 2 shows the model before the exclusion of the next two most strongly associated exposures which also contained seafood.
Table 2. Food items on the tasting menu associated with illness by multivariate logistic regression analysis (n=295, excluding cases with incomplete information for any of the three exposures in the model)
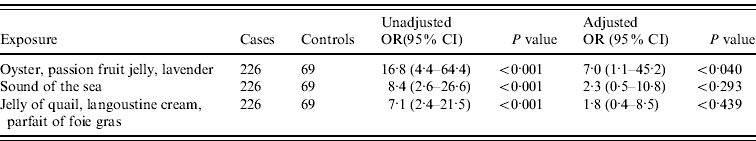
OR, Odds ratio; CI, confidence interval.
Human microbiology
Of the 18 cases from whom stool samples were taken 10 were positive for norovirus, four were negative and no virology results were available for four as they were not submitted for viral testing. The positive norovirus results were obtained from cases in five parties dining at the restaurant on different days during the outbreak. Of the 44 stool samples tested from staff, six were positive for norovirus. Of the staff samples, genogroup II norovirus of strains genotypes GII-2, GII-4 and GII-6 were identified. In the cases (diners), norovirus of both genogroups I and II were identified in two individuals and genogroup II strains were identified in a further seven. These included genotypes GII-3, GII-4 and GII-6.
Food and environmental investigation
Eighty environmental samples were collected over a 3-day period. Of these samples, 26 from high-risk areas were tested for norovirus. High-risk areas included areas where food items were stored, prepared and handled. All were negative for norovirus.
A total of 20 food samples retrieved from the restaurant were sent for bacteriology and virology testing. Escherichia coli and Enterobacteriaceae bacteria were detected in two samples of food retrieved from the restaurant (cooked razor clams and langoustine cream); this is consistent with poor hygiene. The restaurant had started using a new supplier for razor clams (a component of the ‘sound of the sea’ dish) in January of the same year, and a sample of razor clams was obtained directly from this supplier. Norovirus genogroup II was identified from raw razor clams at the limit of detection, signifying low-level contamination with norovirus.
Following the outcome of the analytical epidemiology and the identification of oyster-containing meals as a risk factor, three other norovirus outbreaks linked to this same supplier during January and February 2009 were identified through liaison with the relevant LAs, Centre for Environment, Fisheries and Aquaculture Science (CEFAS) and the Food Standards Agency. Subsequent sampling of oysters harvested from the implicated site in March 2009 tested positive for norovirus (genogroups I and II). Investigations showed no failure in depuration and no specific environmental incident at the implicated harvest site that could account for this. However, it is recognized that depuration is a comparatively ineffective means of removing norovirus because the virus particles become incorporated in the oysters' flesh.
Of the staff members interviewed, 17 reported symptoms of gastrointestinal infection with onset during January or February 2009. Of those staff reporting illness, six reported working while unwell. One of these was from a group of six staff members who later tested positive for norovirus. Nine reported returning to work prior to being asymptomatic for 48 h (against national guidance) and all without negative laboratory tests (against the restaurant's policy). These staff members had a range of jobs in the establishment including handling food and front of house. Illness in staff over this period of time is shown on the epidemic curve in Figure 1.
DISCUSSION
In this study, we describe a very large outbreak of gastrointestinal illness that occurred in diners who ate at a restaurant on a single occasion over a 6-week period, with some secondary spread to household members. Most of the affected diners became symptomatic within 24–48 h of having a meal at the restaurant with the predominant reported symptoms being diarrhoea, vomiting and nausea, lasting a median of 3 days. The symptoms reported by cases, the time between eating at the restaurant and becoming ill, and laboratory investigations identifying norovirus in stool samples taken from diners led us to conclude that norovirus was the causative organism in this outbreak. Restaurant staff with similar symptoms during the same period of time also had laboratory-confirmed norovirus infection. The same genotypes were identified in both diners and staff.
Univariate analysis showed an increased risk of illness associated with consumption of dishes containing molluscan shellfish. Tracing the supply chain of the shellfish allowed testing of both razor clams and oysters from the beds in which the restaurant supplies originated. Tracing was possible from the restaurant's records and batch numbers for supplies. Both were positive for norovirus. Genotyping of the isolates from diners and staff at the restaurant showed multiple different genotypes, a finding consistent with molluscan shellfish-related outbreaks. CEFAS reports that this finding as being typical of oyster-related outbreaks of norovirus [Reference Kageyama14]. The pattern of genotypes in cases following consumption of raw oysters, the presence of norovirus in samples from the same sources of shellfish, albeit not the same batches, and the epidemiological association of oyster consumption with illness makes a compelling case for the role of norovirus infection from oysters in the causal pathway of this outbreak.
The increasing number of cases over time was compatible with a propagated common-source outbreak. Direct infection from oysters was the most likely source of infection leading to this outbreak and contributing to infection until control measures were established. There was some evidence to support other routes of propagation which may have contributed to later cases. The evidence for the razor clams is weaker but we cannot be confident that this food item did not contribute to the outbreak. The complex nature of food preparation in this particular restaurant, with extensive handling of foods, requires excellent food management systems to ensure safety. Two of the 22 food samples taken from the restaurant were contaminated with E. coli and Enterobacteriaceae,reported to be an indicator of a breakdown in food hygiene practices. One of these samples (langoustine cream) was from a dish associated with illness on univariate analysis in the case-control study. Norovirus can also be spread directly from person to person. Several staff members were ill and may have been infectious with norovirus while at work which may have led to contamination of either the environment or the food. It is not possible to rule out food-handler or environment-associated infection of some diners. The stability of norovirus in different environmental conditions means they can remain infectious despite being frozen and even following food being heated to 60°C for 30 min [Reference Cliver, Matsiu, Casteel, Riemann and Cliver15]. The infective dose of norovirus is very low and secondary cases have been shown to appear in foodborne outbreaks following a single exposure [Reference Koopmans16]. This outbreak again highlights the risk associated with consumption of raw or undercooked molluscan shellfish.
The outbreak was reported to the HPA and LA 6 weeks after the putative index case. This delay in reporting to the appropriate statutory authorities resulted in an ongoing risk of exposure to infection for diners. Had the reported illness in diners at the restaurant resulted in the public health authorities being notified earlier then investigations and appropriate interventions could have taken place sooner, potentially avoiding such a high number of cases over such a long period of time.
Foods can generally become contaminated with norovirus via two main routes. First, molluscan shellfish can be contaminated with norovirus originating from human sewage as they feed on particles sweeping through estuarine waters. Current depuration methods may reduce viral load in the mollusc but do not guarantee a safe raw product. As a result norovirus-contaminated oysters have been found to contain a range of norovirus subtypes [Reference Kageyama14, Reference Le Guyader17, Reference Ueki18]. This means that different individuals affected in oyster-associated outbreaks of norovirus infection will tend to excrete a multiplicity of norovirus strains [Reference Le Guyader17, Reference Le Guyader19]. Second, infected food handlers can readily transfer the virus on to foods they prepare. The virus can remain viable and capable of causing illness in those foods that are not subsequently thoroughly cooked, such as salads, canapés and cakes. The more intensively that food is handled the more likely it is to become contaminated by infected food handlers. People eating these foods can then become ill. In addition, any food handlers that continue to work while infected pose a risk to other members of staff.
Norovirus can thus be transmitted to individuals via oysters and/or through other foods contaminated by infected food handlers in those restaurants where contaminated oysters are being served. Staff reported illness throughout this period of time, therefore it is likely that both factors played a role in this outbreak.
The size and duration of this outbreak exceed any other commercial restaurant-associated norovirus outbreaks in the published literature. It is hoped that lessons learned from this outbreak will help to inform future action by restaurateurs especially in early notification to public health authorities once an outbreak is suspected. It is also notable that diners may often choose to inform restaurants directly rather than their doctors or public health authorities. It is important that both diners and restaurants are provided with better information about whom to inform and when to inform once an outbreak of illness is suspected. Updated guidance from the Food Standards Agency for the food industry which includes managing staff illness was published shortly after this outbreak but prior to publication of the report in 2009 [20].
ACKNOWLEDGEMENTS
We acknowledge the contributions of M. Abid, J. Kumbang, T. Hosey, W. Foster, C. Willis, T. Nice, D. Lees and all members of the Incident Control Team to this report.
DECLARATION OF INTEREST
None.





