Anaemia is one of the most common public health problems worldwide. About one-third (32·9 %) of the world’s population was anaemic in 2010 and anaemia was responsible for 68·3 million years lived with disability( Reference Kassebaum, Jasrasaria and Naghavi 1 ). Although a steady decrease in the prevalence of anaemia was observed worldwide since the mid-1990s, the rates of anaemia among women and children younger than 5 years old have changed only slightly( Reference Stevens, Finucane and De-Regil 2 ). Children aged 0–59 months carry the highest burden of anaemia (43 % in 2011)( Reference Kassebaum, Jasrasaria and Naghavi 1 , Reference Stevens, Finucane and De-Regil 2 ). Anaemia has detrimental and possibly irreversible consequences on a young child’s development as it delays brain maturation, leading to impaired motor development and diminished cognitive functioning( Reference Black, Victora and Walker 3 – Reference Beard 5 ). Anaemia is associated with a child’s retarded linear growth( Reference Rocha, Capanema and Netto 6 – Reference Villalpando, Shamah-Levy and Ramirez-Silva 8 ), reduced physical activity( Reference Beard 5 ) and impaired cell-mediated immunity leading to higher susceptibility to infections( Reference Beard 5 , Reference Keusch 9 – Reference Oppenheimer 11 ).
Although there are various underlying causes of anaemia, Fe deficiency is the top cause globally( Reference Kassebaum, Jasrasaria and Naghavi 1 ). According to recent estimates, the proportion of all childhood anaemia resulting from Fe deficiency ranges from 63 % in Europe to 34 % in Africa, while the proportion of severe anaemia attributable to Fe deficiency is over 50 % globally( Reference Stevens, Finucane and De-Regil 2 , Reference Black, Victora and Walker 3 ). Low consumption of meat products is considered to be the major cause of Fe deficiency in young children, especially at weaning age when the Fe requirement is high due to rapid growth and Fe intake from inadequate complementary feeding is low( Reference Black, Allen and Bhutta 4 , Reference Pasricha, Black and Muthayya 12 ). Thus, younger age and insufficient consumption of bioavailable Fe are among well-established determinants of childhood anaemia( Reference Rocha, Capanema and Netto 6 , Reference Villalpando, Shamah-Levy and Ramirez-Silva 8 , Reference Pasricha, Black and Muthayya 12 – Reference Cardoso, Scopel and Muniz 15 ). Other factors commonly related to anaemia in children include male gender, family’s poor socio-economic status (SES), maternal anaemia (especially during pregnancy) and child’s stunted growth( Reference Rocha, Capanema and Netto 6 – Reference Villalpando, Shamah-Levy and Ramirez-Silva 8 , Reference Pasricha, Black and Muthayya 12 , Reference VanBuskirk, Ofosu and Kennedy 14 – Reference Heidkamp, Ngnie-Teta and Ayoya 17 ). In some populations, a substantial proportion of anaemia is attributable to sickle cell disorder, thalassaemia, hookworm, schistosomiasis and malaria( Reference Kassebaum, Jasrasaria and Naghavi 1 ). In some areas, environmental contamination with heavy metals is an increasing concern, inducing anaemia among other health adversities( Reference Tchounwou, Yedjou and Patlolla 18 ).
In Armenia, the Demographic and Health Surveys (ADHS) 2000 and 2005 are the only sources that provided population-based data on anaemia prevalence among 6–59-month-old children in the country. ADHS 2005 identified 36·5 % prevalence of anaemia among children under 5 years of age (under-5s) countrywide (using the HemoCue technique, altitude-adjusted Hb levels and 110 g/l cut-off value)( 19 ). This rate exceeded the ADHS 2000 rate (23·9 %)( 20 ) over 1·5 times, suggesting the possibility of a growing public health problem with childhood anaemia in Armenia. According to the WHO classification, Armenia is in the group of countries having a problem of anaemia of moderate public health significance (a prevalence ranging from 20·0 to 39·9 %)( 21 ). Nevertheless, to our knowledge, no studies exploring risk factors of anaemia among children have been conducted in the country.
The present study was conducted in Talin – a rural region of Armenia which is located on the south-western slope of Mount Aragats, 1200–3300 m above sea level, and is one of the four regions of Aragatsotn Province. It includes one urban and forty rural communities with a total population of 31 600, urban/rural population ratio of 1:5, and children under 18 years of age constitute one-third of the population. Agriculture plays the leading role in the region’s economy. However, the efficiency of agriculture is hampered because of insufficient irrigation systems, unfavourable climate conditions and lack of agricultural machinery. Selling agricultural products is also a major challenge in the region due to poor condition of roads and transportation. According to a 2011 census( 22 ), about 10 % of the population in Aragatsotn Province is employed and 27 % is self-employed in farming, while over 50 % is dependant or retired. The prevalence of anaemia among 6–59-month-old children in the province, according to ADHS, was stable – 25·5 % in 2000 and 26·5 % in 2005. However, the rate of severe anaemia (Hb below 70 g/l) among them increased from 1·4 % in 2000 to 7·1 % in 2005( 19 , 20 ).
Since 2008, World Vision (WV) Armenia has targeted the majority of communities in Talin region with activities in the sectors of economic development, HIV/AIDS prevention, education and nutrition. The activities were carried out through community involvement and local capacity building. Interventions to improve the nutritional status of women and children aimed to promote healthy nutrition in general and included seminars for women on child care and nutrition, dissemination of education materials on these topics, establishing Mother Support Groups, promoting locally available healthy foods and capacity building of primary health-care providers. Some of the targeted communities received the full set of five interventions listed above, while others received a partial set of two to four interventions. Neither the prevalence of anaemia nor its risk factors were ever studied in the region and no specific anaemia-reduction interventions were carried out. The aim of the present study was to measure the prevalence of anaemia among under-5s residing in these communities and identify the determinants of anaemia. The results of the study could help WV and other stakeholders to expand their health promotion activities to include anaemia reduction.
Methods
Study design and participants
The study participants were children aged 0–59 months residing in WV target communities of Talin region, Aragatsotn Province. A cross-sectional study design was applied to identify the prevalence of anaemia among them. The study utilized a probability-proportional-to-size (under-5s population size) cluster sampling design to select communities via systematic random sampling. Then study participants were randomly selected from enumerated ambulatory lists of under-5s in the selected communities. The study used the statistical software package STATA version 10 for sample size calculation, applying the formula for one-sample comparison of proportion (taken as 0·265( 19 )) to a hypothesized value (0·215) to detect a difference of 5 % with 0·8 power and type 1 error of 0·05. An estimated cluster design effect coefficient of 1·25 was applied to the calculated sample size of 587, increasing it to 734, which was further increased to 800 anticipating ~10 % non-response. The total number of under-5s in the targeted communities was 2080, meaning that the cross-sectional study was designed to include over one-third of the eligible population. Selected children were invited to local ambulatories and underwent Hb measurement in capillary blood taken from a finger. The study used the HemoCue Hb 201+ analyser to measure blood Hb concentrations, because it provides quickness of measurement, ease of use and maintenance combined with proven precision and accuracy( Reference Bäck, Magnusson and Norlund 23 ). The HemoCue system is based on the cyanmethaemoglobin method – the reference laboratory method for Hb measurement used for standardization of other methods and is generally recommended for use in population surveys as a stable and reliable method in field settings( 21 ). Trained field teams, each comprised of a paediatrician and a laboratory nurse, conducted the measurements. As the majority of communities in Talin region are more than 1000 m above sea level, Hb concentrations obtained through HemoCue were further adjusted for the area’s altitude applying the adjustment values recommended by the WHO( 21 ). Cut-off values to diagnose anaemia in children aged under 5 years were altitude-adjusted Hb level <70 g/l for severe, 70–99 g/l for moderate and 100–109 g/l for mild anaemia( 21 ). In addition, the field teams conducted weight and height measurements of each child, reviewed his/her ambulatory chart and abstracted data on the child’s age, gender, weight and length at birth, term of birth and any chronic conditions diagnosed.
After the cross-sectional study, the research team conducted a case–control study to identify determinants of anaemia among children aged 0–59 months in Talin region. The decision to conduct a case–control study was based on both feasibility and efficiency considerations, as the study was limited in time and resources to conduct interviews with mothers of all children included in the cross-sectional study sample. A case/control rate of 1:1 was applied. All children identified as having Hb level less than 105 g/l were included in the case–control study as cases. The study team randomly selected controls from the pool of studied children identified as having Hb level of 110 g/l or more, using the Select Random Sample of Cases function from the SPSS menu. The research team purposefully avoided age and gender matching of cases and controls, as matched characteristics cannot be investigated further, while one of the aims of the study was to identify whether age and/or gender were independent determinants of anaemia, so that target groups for anaemia-reduction interventions could be correctly defined by age and gender. Hb cut-off values of less than 105 g/l to select cases and 110 g/l or more to select controls were applied with the intention to have definite cases with anaemia and controls with no anaemia to minimize misclassification bias in the case–control study sample. A number of studies have recommended and used Hb values of 105 g/l or less as more accurate cut-offs to diagnose anaemia in infants and young children( Reference Meyerovitch, Sherf and Antebi 16 , Reference Domellof, Dewey and Lonnerdal 24 – Reference Sherriff, Emond and Hawkins 29 ). Trained interviewers, blind to the anaemia status of children, conducted face-to-face interviews with mothers of cases and controls, using a structured questionnaire. Data collection for the prevalence study took place in October–November 2013 and for the case–control study in December 2013.
Variables
The dependent variable of the case–control study was the presence of anaemia in a child aged 0–59 months. Independent variables included potential risk or confounding factors of childhood anaemia( Reference Sanou and Ngnie-Teta 30 ) and were grouped into the following subgroups: child’s characteristics, maternal/birth-related characteristics, child’s nutrition patterns and living standards/environment. Child’s characteristics included age, gender, birth length and weight, any diagnosis with intestinal worms during the last year, any significant blood loss in recent months and any recent lengthy diarrhoea. Maternal/birth-related characteristics included mother’s age (at the child’s delivery), height, education, employment, anaemia during pregnancy, rhesus conflict between the mother and child, child’s birth order, delivery term, delivery mode, inter-birth interval before the child’s birth and mother’s child-care knowledge score. Child’s nutrition patterns included breast-feeding initiation time (within the first hour after birth or later), duration of exclusive and any breast-feeding, age at introduction of tea, juice and cow’s milk, food diversity score, number of meals per day and consumption of cow’s milk and meat in the last 24 h. Living standards and environment included child’s family size, SES coefficient, household heating mode (heating with dung cakes v. hot water, electric, gas/oil or coal/wood heating), number of times the child’s hands were washed per day, use of soap during hand-washing and whether the complete set of WV interventions was carried out in the community.
Most independent variables were measured based on single items, with exception of the three summative scores developed by the authors: child’s food diversity score, mother’s child-care knowledge score and family’s SES coefficient. Child’s food diversity score (with a range of 0–13) was a cumulative number of the food types (of thirteen adapted from the food category list used in ADHS questionnaire( 31 )) the child consumed during the last 24 h, as reported by the mother. Mother’s child-care knowledge score (with a range of 0–10) was a sum of correct replies to ten questions on different aspects of child care. For family’s SES coefficient, the study used SES regression coefficients for the first factor (explaining 40 % of the variance in the sample) from the principal component analysis with the items on family’s average monthly expenditures, perceived living standards and possession of various convenience/luxury items. The online supplementary material presents the items used to construct the scores and the SES coefficient.
Analysis
The research team used the cross-sectional study sample to identify the prevalence of anaemia. The characteristics of those with and without anaemia in the case–control study sample were analysed descriptively and compared statistically using Student’s t test (for normally distributed variables) or the Mann–Whitney U test (for non-normally distributed variables) for means and the χ 2 test for proportions. This was followed with univariate and multivariate logistic regression analyses with the outcome of anaemia status. The research team dichotomized all the nominal/ordinal variables before entering them into logistic regression analyses to enhance the interpretability of the findings. The continuous variables were treated as continuous after checking their linearity on the logistic scale with the outcome of anaemia status( Reference Hosmer and Lemeshow 32 ). The multivariate analyses included all the variables associated with the outcome at the P<0·25 level in the univariate analysis, as recommended by Hosmer and Lemeshow( Reference Hosmer and Lemeshow 32 ). During fitting the models, the research team entered independent variables into multivariate analysis in different combinations using the enter function. The fit of the final logistic regression model was tested via pseudo R 2, the Hosmer–Lemeshow goodness-of-fit test and the area under the receiver-operating characteristic curve. The research team fitted also a multiple linear regression model with the outcome of Hb concentration in capillary blood to take advantage of using the whole variability of the continuous outcome variable. All the variables included in the final logistic or linear regression models were checked for interaction. Collinearity issues between these variables were also checked by applying variance inflation factor statistics. The analyses were conducted using the STATA version 10 and SPSS version 11 statistical software packages.
Results
Overall, 729 children younger than 5 years (mean age 30·5 (sd 16·3) months) underwent Hb measurement. Of them, 404 (55·4 %) were boys and 325 (44·6 %) girls. The altitude-adjusted mean Hb level among the studied children was 114·3 (sd 14·1) g/l (range 60–152 g/l). The prevalence of anaemia in this sample, defined as altitude-adjusted blood Hb level below 110 g/l, was 32·4 (95 % CI 29·0, 35·9) %. Of those identified as anaemic, 17·7 % had mild anaemia, 13·9 % had moderate and 0·8 % had severe anaemia. The highest prevalence of anaemia (67·9 %) was detected among 6–12-month-old children, with the next highest rates in the age groups of 12–18 months (52·6 %) and 0–6 months (51·1 %). After 18 months of age, a clear decreasing tendency in the prevalence of anaemia was observed (Table 1). The proportion of moderate/severe anaemia was the highest among children aged 6–12 months.
Table 1 Prevalence of anaemia (Hb below 110 g/l) among children aged 0–59 months in Talin region, Aragatsotn Province, Armenia, 2013

N, number of children in each age group; Hb, altitude-adjusted Hb level.
The case–control study sample included 101 cases of anaemia with altitude-adjusted Hb level of less than 105 g/l and eighty-seven healthy controlsFootnote * with Hb concentration of 110 g/l or more in capillary blood. There were no refusals for the interviews. Table 2 depicts the distribution of independent variables among anaemic children and controls and the results of statistical comparisons between them. Children with anaemia were significantly younger and had lower birth weight and shorter birth length (all P<0·001). Mothers of cases and controls were not significantly different in their age at the child’s delivery (25·4 and 24·9 years old, respectively), education (39·6 % and 36·8 % with graduate/higher education, respectively) or employment (17·8 % and 10·5 %, respectively). The proportion of preterm deliveries (P=0·036) and caesarean sections (P=0·037) with the given child was significantly higher among mothers of anaemic children compared with mothers of controls. The majority of nutrition-specific variables were significantly positively associated with child’s anaemia status during univariate comparisons, including late initiation (after the first hour of life; P=0·063) and shorter duration of breast-feeding (P=0·002), earlier introduction of cow’s milk (P=0·025) and juice (P=0·040) into the child’s diet, higher child’s meal frequency per day (P=0·015) and lower food diversity score (P<0·001), and presence of cow’s milk (P=0·034) and absence of any meat (P=0·005) in the child’s diet in the last 24 h. No difference was detected between the groups in the number of family members (6·5 for cases and 6·3 for controls) or child’s birth order (1·9 for both). Of the variables on living standards and environment, use of dung cakes for heating the living quarters was positively associated with child’s anaemia (P=0·026), and living in communities where the complete set of WV interventions was carried out was inversely related to anaemia (P<0·001).
Table 2 Distribution of selected characteristicsFootnote * between children aged 0–59 months with anaemia and controls in Talin region, Aragatsotn Province, Armenia, 2013
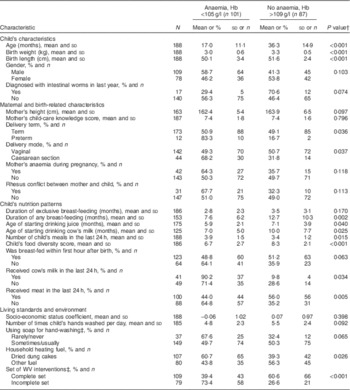
N, number of valid cases; Hb, altitude-adjusted Hb level; WV, World Vision.
* The groups were not significantly different in variables of child’s recent blood loss, mother’s age at delivery, education, employment, inter-birth interval, child’s birth order, age of starting drinking tea and number of family members.
† Student’s t test or the Mann–Whitney U test for means and the χ 2 test for proportions (two-sided P values).
‡ The variable was dichotomized.
The final logistic regression model identified seven independent determinants of anaemia among 0–59-month-old children: child’s age, gender, length at birth, maternal anaemia during pregnancy, child’s meal frequency per day, burning dung cakes for heating and whether the complete set of WV interventions was carried out in the child’s community (Table 3). After controlling for the remaining significant variables, each one month increase in child’s age was associated with a lower chance (OR=0·89; 95 % CI 0·86, 0·92) for the child to be anaemic. Boys were more likely (OR=3·34; 95 % CI 1·36, 8·17) to develop anaemia than girls. Each additional centimetre of child’s birth length was associated with a decreased chance (OR=0·80; 95 % CI 0·67, 0·95) for him/her to develop anaemia. Mother’s anaemia during pregnancy was associated with increased likelihood (OR=4·81; 95 % CI 1·51, 15·39) for the child to develop anaemia. Feeding a child one additional time per day was associated with a lower chance (OR=0·68; 95 % CI 0·48, 0·96) of anaemia. Using dung cakes for heating was associated with a greater chance (OR=3·00; 95 % CI 1·25, 6·96) for a child to develop anaemia. Residing in a community where the complete set of WV interventions was carried out was associated with a lower likelihood (OR=0·28; 95 % CI 0·11, 0·70) for a child to be anaemic. The model reached very good fit statistics (Table 3).
Table 3 Logistic regression model of determinants of anaemia among children aged 0–59 months in Talin region, Aragatsotn Province, Armenia (valid N 183)

WV, World Vision; ROC, receiver-operating characteristic.
* The variable was dichotomized at a threshold level reflecting the pattern of its association with the outcome on the logistic scale.
The final linear regression model with the outcome of blood Hb level identified a slightly different set of independent predictors of childhood anaemia: child’s gender and birth length lost their significance, while usage of any meat in the child’s diet in the last 24 h became a marginally significant predictor of child’s anaemia status with a high coefficient (4·1). The remaining determinants of childhood anaemia (child’s age, number of meals per day, maternal anaemia during pregnancy, using dung cakes for heating and whether the complete set of WV interventions was carried out in the community) remained significant in the final linear regression model (Table 4). The model explained over 43 % of the variability of the outcome. No interactions and no issues with collinearity were identified between the variables included in the final models (all variance inflation factor values were below 1·3).
Table 4 Linear regression model for altitude-adjusted Hb level among children aged 0–59 months in Talin region, Aragatsotn Province, Armenia (valid N 184, R 2=0·431)

WV, World Vision; r, correlation coefficient.
* The variable was dichotomized.
Discussion
The present study identified altitude-adjusted mean Hb level of 114·3 g/l among children aged 0–59 months residing in Talin region, Aragatsotn Province, Armenia. This level is well below the mean for under-5s from high-income countries (123 g/l), is similar to that for children from Central Asia, the Middle East and North Africa (114 g/l) and is slightly above the overall mean for children worldwide (111 g/l)( Reference Stevens, Finucane and De-Regil 2 ). According to the most recent available data from ADHS 2005 on the prevalence of anaemia in Aragatsotn Province, the proportion of 6–59-month-old children with anaemia was 26·5 % (with 16·3 % mild, 3·1 % moderate and 7·1 % severe cases)( 19 ). For the same age group (6–59 months old), the prevalence of anaemia identified in the current study was 31·2 % (with 16·7 % mild, 13·6 % moderate and 0·9 % severe cases), which is not statistically significantly different from the ADHS 2005 rate, indicating that the prevalence of anaemia among children under 5 years old has been relatively stable in this region since 2005.
In the current study, the highest prevalence of anaemia was observed among children under 18 months old with a peak among those aged 6–12 months, which is consistent with reports indicating higher vulnerability to anaemia of children in the age group under 24 months with the highest prevalence during late infancy( Reference Rocha, Capanema and Netto 6 , Reference Villalpando, Shamah-Levy and Ramirez-Silva 8 , Reference Cardoso, Scopel and Muniz 15 , Reference Meyerovitch, Sherf and Antebi 16 ). The most common explanation for this phenomenon is that infants’ Fe stores are getting depleted at this period because of rapid growth, as growth velocity correlates significantly with the extent of Fe deficiency( Reference Thorsdottir, Gunnarsson and Atladottir 28 , Reference Sherriff, Emond and Hawkins 29 ). Meanwhile, dietary intake of Fe during the first 2 years of life is usually lower than recommended, especially in low-/middle-income countries( Reference Villalpando, Shamah-Levy and Ramirez-Silva 8 , Reference Pasricha, Black and Muthayya 12 ). It is noteworthy that the observed rate of anaemia among 6–12-month-old children in Talin region (67·9 %) is comparable to the rates of anaemia among 6–9-month-old children in the most disadvantaged areas of the globe, e.g. sub-Saharan Africa (64–93 %) and Southeast Asia (70–85 %)( Reference Chaparro 33 ).
The present study identified also an alarmingly high prevalence of anaemia among the youngest age group of children in Talin region. Over half of the children aged 0–6 months had blood Hb level below 110 g/l. This is worrying, as under optimal conditions with maternal Fe status, gestational age, birth size, umbilical cord clamping time and breast-feeding, the Fe stores should be adequate for at least the first 6–8 months of life( Reference Chaparro 33 ). Unless caused by reasons other than Fe deficiency, the high prevalence of anaemia in the youngest age group of children indicates the possible shortcomings with perinatal care and infant feeding practices in this region. In particular, lack of Fe supplementation prenatally, immediate umbilical cord clamping and early introduction of cow’s milk into the child’s diet could be the main risky behaviours associated with the high prevalence of anaemia among children under 6 months old( Reference Chaparro 33 – Reference Preziosi, Prual and Galan 35 ).
The current study identified the following independent predictors of anaemia among under-5s in Talin region: child’s age, gender, maternal anaemia during pregnancy, child’s birth length, number of child’s meals per day, presence of meat in the child’s diet, using dung cakes for heating and whether the whole set of WV interventions was carried out in the community. Some of these factors, like younger age and male gender, are well-established non-modifiable predictors of childhood anaemia( Reference Rocha, Capanema and Netto 6 , Reference Villalpando, Shamah-Levy and Ramirez-Silva 8 , Reference Pasricha, Black and Muthayya 12 – Reference Cardoso, Scopel and Muniz 15 , Reference Heidkamp, Ngnie-Teta and Ayoya 17 , Reference Emond, Hawkins and Pennock 25 , Reference Sherriff, Emond and Hawkins 29 ). While the reasons for higher vulnerability of younger children to Fe-deficient anaemia are well known, the reason for boys to be more susceptible to Fe deficiency compared with girls is a matter of debate. Various mechanisms are suggested to explain this difference, including metabolic differences in Fe absorption and losses, differences in proportions of lean and fat tissues in the body, and hormonal differences( Reference Chaparro 33 ).
Maternal anaemia during pregnancy was a strong predictor of childhood anaemia in the present study, which is consistent with other reports( Reference Meyerovitch, Sherf and Antebi 16 , Reference Kilbride, Baker and Parapia 26 , Reference Chaparro 33 ). Although the active transfer of Fe to the fetus is maximized in Fe-deficient pregnant women as a compensatory mechanism to protect the fetus, and the findings on the immediate effect of maternal Fe deficiency on the newborn’s Fe status are conflicting( Reference Kilbride, Baker and Parapia 26 , Reference Kumar, Rai and Basu 36 , Reference El-Farrash, Ismail and Nada 37 ), longitudinal studies have demonstrated that maternal anaemia can have a long-term effect on the child’s Fe stores resulting in their earlier depletion( Reference Kilbride, Baker and Parapia 26 , Reference Chaparro 33 ). Lower concentration of Fe in the breast milk of severely Fe-deficient women could be another mechanism contributing to this depletion( Reference Kumar, Rai and Basu 36 , Reference El-Farrash, Ismail and Nada 37 ).
Birth length was a protective factor for child’s anaemia in the current study that mediated the influence of birth weight on anaemia status. Other studies report an independent positive relationship between child’s birth weight and ferritin concentration( Reference Emond, Hawkins and Pennock 25 , Reference Sherriff, Emond and Hawkins 29 ). A double-blinded clinical trial of Fe supplementation during pregnancy identified higher mean birth length of neonates of Fe-supplemented mothers, providing evidence for a positive relationship between birth length and maternal Fe status( Reference Preziosi, Prual and Galan 35 ). In the current study, birth length remained a significant predictor of child’s anaemia even after controlling for maternal anaemia during pregnancy. However, the information on the latter could be under-reported because of recall bias or mother’s ignorance of her anaemia status during pregnancy, especially if she had mild anaemia or did not test prenatally.
Child’s meal frequency per day and inclusion of meat into the child’s diet in the last 24 h were also independently related to child’s anaemia status. More frequent meals per day and meat consumption both reduced the chance of anaemia in a child. The frequency of having meals at home was a predictor of anaemia among 2–5-year-old children in a large-scale study conducted in poor areas of China( Reference Li, Yu and Liu 38 ). Low consumption of meat is considered to be the major cause of Fe-deficiency anaemia, especially among the poor( Reference Black, Allen and Bhutta 4 ). A number of studies have found a positive association between meat intake and child’s Fe status( Reference Michaelsen, Milman and Samuelson 27 , Reference Thorsdottir, Gunnarsson and Atladottir 28 , Reference Engelmann, Sandstrom and Michaelsen 39 ). Meat is not only a source of highly bioavailable haem Fe itself, but also acts as an enhancer of absorption of both haem and non-haem Fe from other sources, drastically increasing the overall bioavailability of dietary Fe( Reference Hallberg, Hoppe and Andersson 40 ). Thus, inadequate consumption of meat during the period of introduction of solid foods and thereafter because of poverty or local diet specifics poses a child to a real danger of developing anaemia.
Using biomass fuel (dung cakes) for heating was another independent predictor of childhood anaemia in the present study. This is an interesting finding in light of a large-scale study conducted among 0–35-month-old children in India, which found a strong dose–response relationship between the use of inefficient biomass fuel (including dung cakes) for heating and cooking and the risk of anaemia in children( Reference Mishra and Retherford 41 ). Another recent large-scale study also identified an independent association between anaemia among under-5s and the use of unclean fuel in the household( Reference Baranwal, Baranwal and Roy 42 ). According to the WHO, dried dung and crop waste are at the bottom of the ‘energy ladder’ in terms of being the cheapest but the most inefficient and polluting domestic fuel( 43 ). Meanwhile, indoor air pollution from the use of unclean fuel was found to disproportionately affect women and young children, as women spend many hours at the cooking devices, often keeping their young children indoors with them( Reference Fullerton, Bruce and Gordon 44 ). Similar to other studies( Reference Mishra and Retherford 41 , Reference Baranwal, Baranwal and Roy 42 ), the standard of living/wealth index was not independently related to child’s anaemia status in the current study, suggesting that the mechanism underlying the relationship between childhood anaemia and the use of unclean domestic fuel is not explained by poverty alone.
Living in a community where the complete set of WV interventions was implemented also significantly reduced the likelihood for a child to be anaemic. This finding could indicate the effectiveness of the complete v. incomplete set of WV interventions in these communities. Nevertheless, these communities could be different from each other as the communities that received the complete set of WV interventions were mainly larger communities or those with easier access to the roads to the capital city, while those that received an incomplete set of interventions were smaller and more remote. Hence, this variable could also reflect differences between the communities other than the set of WV interventions.
The main strength of the present study was the accurate measurement of the outcome variable (i.e. blood Hb level) using one of the most advanced/recommended equipments (i.e. the HemoCue 201+ analyser), which resulted in realistic prevalence estimates of anaemia among under-5s in Talin region and minimized the possibility of misclassification bias during the case–control study. The main limitation of the study was its coverage of communities located in one region, Talin, which limited the generalizability of the study findings to other regions of Armenia. Other limitations included the lack of direct tests to measure the Fe status of children, the relatively small sample size of the case–control study and the self-reported nature of the majority of independent variables included in the case–control study, especially those related to child’s diet, making them subject to reporting and recall biases. As the study design did not allow selecting incident cases of anaemia, we selected prevalent cases, which in theory could make it impossible to identify temporal relationships between the outcome and associated factors. However, the nature of most risk factors found in the study (e.g. mother’s pregnancy anaemia, child’s age, gender, birth length, biomass fuel use, etc.) excludes the possibility of a reverse temporal relationship between them and child’s anaemia status. Also, the present study was the first attempt to explore determinants of anaemia among children from rural Armenia and resulted in a number of important findings that could be used by policy makers in designing interventions to reduce childhood anaemia in the country.
The identified high prevalence of anaemia among infants, with an onset in early infancy and a peak at 6–12 months of age, is disturbing given the possibly irreversible consequences of early-onset anaemia on children’s development and long-term physical and cognitive performance( Reference Black, Victora and Walker 3 – Reference Beard 5 ). This reality calls for immediate action with carefully designed and well-targeted interventions. To prevent early-onset Fe deficiency in infants, researchers are uniform in recommending to scale up strategies with known effectiveness: Fe supplementation during pregnancy, delayed cord clamping, immediate initiation and exclusive breast-feeding for 6 months, and timely and appropriate complementary feeding after 6 months( Reference Chaparro 33 , Reference Stoltzfus 45 ). It is very important that children receive an adequate amount of bioavailable Fe starting from the first days of the initiation of complementary feeding. This requirement could be met by adding meat to a child’s daily diet. Hallberg et al. found a drastic increase in both non-haem and haem Fe absorption from a gruel (to a level of the daily Fe requirement of a 12-month-old infant) after adding a powdered meat to it( Reference Hallberg, Hoppe and Andersson 40 ).
An alternative way of providing additional Fe to Fe-deplete individuals is to use Fe supplements in a form of pills or syrups( 46 ). This strategy requires identification of the Fe status of the recipient first, as Fe supplementation, while beneficial for Fe-deficient children, may cause harm to Fe-replete children by interfering with their optimal growth, causing oxidative damage to the tissues and increasing their susceptibility to infections, particularly malaria, tuberculosis and intestinal infections( Reference Collins 10 , Reference Lannotti, Tielsch and Black 47 , Reference Domellof 48 ). Thus, this strategy should selectively target Fe-deficient children( Reference Stoltzfus 45 , Reference Lannotti, Tielsch and Black 47 , Reference Domellof 48 ). Staple food fortification with Fe is another way to reduce Fe deficiency, but the main requirement for this strategy is that the fortified food should be consumed in sufficiently large amounts by the vulnerable population groups( 46 ). Therefore, to prevent Fe deficiency in early childhood, Fe is usually added to commercial food items for children. However, these foods could be inaccessible and/or unaffordable for poor rural families. Alternatively, a promising approach could be to use micronutrient powders for home-made complementary food fortification, found to be successful in a number of studies( Reference Lundeen, Schueth and Toktobaev 49 , Reference Troesch, van Stuijvenberg and Smuts 50 ), but the success of this approach depends heavily on mothers’ compliance.
Given the restrictions of Fe supplementation strategies, adequate diet and healthy environment remain the cornerstones of preventing Fe deficiency in children( 46 , Reference Hurrell, Ranum and de Pee 51 ). Early introduction of cow’s milk into a child’s diet, especially as a substitute for breast milk, a practice rather widespread in rural Armenia, should be avoided for its proven inhibitory influence on Fe absorption from other foods and for its ability to cause small intestinal haemorrhages because of inducing allergic inflammation of the intestinal mucosa( Reference Oliveira and Osorio 34 ). There are a number of important interventions that could be undertaken in rural Armenia to eliminate the modifiable risk factors of childhood anaemia, including prevention and treatment of anaemia during pregnancy, adequate complementary feeding of children with inclusion of meat into a child’s daily diet, and reduction of women’s and children’s exposure to inefficient biomass fuel smoke. As most of the risk factors of childhood anaemia identified in the current study are frequently reported to be determinants of anaemia in other countries as well( Reference Meyerovitch, Sherf and Antebi 16 , Reference Kilbride, Baker and Parapia 26 , Reference Michaelsen, Milman and Samuelson 27 , Reference Li, Yu and Liu 38 , Reference Engelmann, Sandstrom and Michaelsen 39 ), the present study’s findings could be applicable to other countries, especially those with similar economic background. In this respect, the finding on the independent relationship between using biomass fuel and childhood anaemia is of particular interest, as a considerable proportion of households not only in rural Armenia but also in rural areas in other low-/middle-income countries use this type of fuel for heating and cooking, and yet only a few studies( Reference Mishra and Retherford 41 , Reference Baranwal, Baranwal and Roy 42 ) highlight the association between this behaviour and child’s anaemia status. Further research specifically targeting 0–6-month-old infants is recommended to identify the predictors of early-onset anaemia in rural Armenia.
Acknowledgements
Acknowledgements: The authors are grateful to Dr Zaruhi Bakalyan for her input during the preliminary literature review for this paper and WV Armenia staff members Drs Naira Gharakhanyan, Ruzanna Manukyan and Arax Hovhannesyan for their contribution to the initial discussions of the project. The authors also thank Dr Tsovinar Harutyunyan at the American University of Armenia for her contribution to the project. The authors express their gratitude to the Ministry of Health of Armenia and the field workers and participants of the study. Financial support: World Vision Middle East and Eastern Europe Regional Office funded this study. Conflict of interest: V.S. is employed in the aforementioned agency. The remaining authors declare no conflict of interest. Authorship: All authors participated in conceptualizing and designing the study. A.D. acquired the data, performed the analysis and drafted the manuscript. V.P. and K.H. critically revised the manuscript for important intellectual content. All authors have read and approved the final manuscript. Ethics of human subject participation: The study was conducted according to the guidelines laid down in the Declaration of Helsinki. The Institutional Review Board of the American University of Armenia approved the study protocol. Mothers/legal guardians of children provided written consent to participate.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1368980015002451







