The increased overweight and obesity prevalence observed in industrialised countries, which nowadays affects two-thirds of their population, determines a higher risk of developing type 2 diabetes mellitus and cardiovascular disease-related death.
Changing lifestyle habits is still the main cornerstone of the therapy for obesity, but improvement of cardiometabolic parameters as hyperglycaemia, insulin resistance, abdominal obesity and atherogenic dyslipidaemia must be major targets, as well as weight reduction.
Moderate weight loss (approximately 5–10% of body weight) by lifestyle changes improves obesity-related comorbidities. Unfortunately, this medical approach is not long lasting and weight regain is often seen. Drugs that prevent weight regain appear necessary in obesity treatment.
Safety on anti-obesity agents have historically been of concern due to severe side-effects that contraindicated their use. Actually, sibutramine and orlistat are the only authorised anti-obesity drugs in Europe. Efficacy and safety of other molecules are currently being evaluated on phase II–III clinical trialsReference Halford1, but only one of them, rimonabant, will be a real therapeutical option in the next months.
Safety and tolerability of these drugs, as well as their efficacy on cardiometabolic risk factors will be analysed in this article.
Sibutramine
The mode of action of sibutramine is centrally mediated by a serotonin and epinephrine reuptake inhibition, which enhances satiety and decreases caloric intake. Sibutramine has shown marked thermogenic properties in laboratory animals but is not significant in human beings.
The usefulness of sibutramine in obesity treatment has been widely demonstrated in multiple prospective randomised trials. Probably the most significant is the STORM study (Sibutramine Trial of Obesity Reduction and Maintenance)Reference James, Astrup, Finer, Hilsted, Kopelman and Rossner2 that demonstrated its utility on weight reduction as well as on weight loss maintenance. A higher weight loss in sibutramine-treated obese patients of approximately 4.5 kg compared to placebo has been show in long-term studies in different meta-analysesReference Padwal, Li and Lau3, Reference Li, Maglione, Tu, Mojica, Arterburn and Shugarman4. Far beyond its efficacy, effects in selective obese groups and clinical use are discussed below.
Obese adolescents
Some clinical trials using sibutramine (10 or 15 mg day−1) have shown promising results achieving weight loss in obese adolescentsReference Godoy-Matos, Carraro, Vieira, Oliveira, Guedes and Mattos5–Reference Reisler, Tauber, Afriat, Bortnik and Goldman7, but the samples were very small and gave rise to some safety concerns about the 15 mg day−1 dose.
Recently, a multicentre randomised trial from the Sibutramine Adolescent Study GroupReference Berkowitz, Fujikova, Daniels, Hopping, Owen and Perry8, including 368 adolescent treated with sibutramine uptitrated to 15 mg during 1 year, confirmed a statistically significant decrease in body mass index (BMI) and weight in the sibutramine group vs. placebo. In relation to adverse events, only small, although statistically significant, differences in blood pressure and heart rate were described, similar to observations previously reported in adults.
Therefore, sibutramine could become an acceptable additional therapeutic option in obese adolescents, considering the same recommendations as in adults treated with sibutramine, to monitor blood pressure and pulse rate.
Binge eating
Efficacy of sibutramine on binge eating disorder has been recently evaluated in controlled clinical trials. Two of them showed positive results on three of the final outcomes after 12 weeks treatment with sibutramine (10 mg day−1): weight reduction, decrease in binge eating episodes and psychiatric symptomsReference Appolinario, Bacaltchuk, Sichieri, Claudino, Godoy-Matos and Morgan9, Reference Milano, Petrella, Casella, Capasso, Carrino and Milano10. Subclinical binge eating and clinical results with sibutramine treatment was evaluated on the third trial where a greater weight reduction was observed when compared to placebo, but failed to demonstrate improvement on the psychiatric symptomsReference Bauer, Fischer and Keller11.
Very-low-calorie diets
Very-low-calorie diets (VLCDs) are used to promote short-term weight loss in obese patients. However, long-term maintenance of weight loss is generally poor. Weight loss achieved after a 3-month VLCD is more effectively improved and maintained with sibutramine plus a diet and exercise programme than with placebo over a follow-up period of 12–18 monthsReference Apfelbaum, Yague, Ziegles, Hanotin, Thomas and Leutenegger12, Reference Mathus-Vliegen13 (Table 1). These results agree with those obtained in the STORM studyReference James, Astrup, Finer, Hilsted, Kopelman and Rossner2, which demonstrated maintenance of weight reduction with sibutramine than with a conventional dietetic approach.
Table 1 Randomised trials: very-low-calorie-diets plus sibutramine

Sib – sibutramine; Pl – placebo.
Type 2 diabetes mellitus
Type 2 diabetes obese patients must be specially considered due to their increased morbidity and mortality and additional difficulties to obtain weight reduction. Sibutramine usefulness in this group of patients has recently been evaluated on different trialsReference Vettor, Serra, Fabris, Pagano and Federspil14, Reference Norris, Zhang, Avenell, Gregg, Schmid and Lau15. More than 50% of diabetic subjects receiving sibutramine achieve >5% weight loss and over 20% achieved >10% weight loss. As expected, higher weight losses are related to an improvement on glycosylated haemoglobin, triglycerides and high-density lipoprotein (HDL) cholesterol levels. Moderate but significant higher diastolic blood pressure and heart rate were observed as side-effects, recommending careful evaluation and control of these parameters in this subgroup of obese patients.
Metabolic effects
Sibutramine exerted favourable effects on lipids in most trials, especially on HDL cholesterol and triglycerides, as well as on the total : HDL cholesterol ratio. Furthermore, this drug seems to favourably influence adipocytokines; it reduces serum leptin and resistin levels and increases adiponectin levelsReference Filippatos, Kiortis, Liberopoulos, Mikhailidis and Elisaf16.
Safety and adverse events
Long-term sibutramine treatments have revealed beneficial effects on the risk to develop primary pulmonary hypertension and heart valve dysfunction, as observed in previous serotonin agonist drugs like d-fenfluramine.
Controversy still remains regarding the impact of changes on blood pressure and heart rate, mainly because hypertension is one of the obesity-associated diseases that is expected to improve after obesity therapy.
Sibutramine increases systolic and diastolic blood pressure by 2–3 and 1–2 mmHg, respectively, and medium heart rate augments by 4–5 beats per minuteReference Jordan, Scholze, Matiba, Wirth, Hauner and Sharma17, Reference Kim, Lee, Jee and Nam18. Anyway, the potential benefits of weight loss outweighed the small increases in heart rate and blood pressure, as shown in the largest study that included 2225 subjects with cardiovascular risk factors, from a primary care settingReference Gaciong and Placha19.
Enlarging of the QT segment and arrhythmia has been recently reported in patients treated with sibutramineReference Harrison-Woolrych, Clark, Hill, Rees and Skinner20. Caution must be taken to avoid the use of sibutramine in patients with long QT syndrome, or treated with drugs related to the increase in the QT segment until safety data are available.
Questions concerning cardiovascular outcomes will be clarified with the SCOUT (Sibutramine Cardiovascular Morbidity/Mortality Outcomes Trial) study results. This clinical trial has been performed over 9000 cardiovascular risk obese patients randomised to sibutramine or placebo plus lifestyle changes, followed up for 5 years. Primary outcomes include the incidence of non-fatal myocardial infarction, non-fatal stroke, resuscitated cardiac arrest and cardiovascular death.
The most common side-effects associated with sibutramine use are mild and usually well tolerated: mouth dryness, constipation, headache and insomnia. Patient must withdraw treatment if mean blood pressure increases over 10 mmHg or if heart rate augments by more than 10 beats per minute. Although the evidence of increased risk for cardiovascular or cerebrovascular events has not yet been clearly demonstrated, sibutramine should not be given to patients with uncontrolled hypertension or to patients with a known heart disease or stroke.
Orlistat
Orlistat is a potent inhibitor of pancreatic and gastric lipases that acts locally in the gut lumen and is minimally absorbed. Orlistat inhibits dietary triglycerides hydrolysis by 30%; hence they pass into the stool, thus reducing total calorie intake.
A mean weight loss of 2.8–3.2 kg reduction additional to placebo, as well as significant improvement of blood pressure, lipids and metabolic control in diabetes have been described in meta-analysisReference O’Meara, Riemsma, Shirran, Mather and ter Riet21–Reference Hutton and Fergusson23. Pooled data from randomised trials and from the open-label study X-PERTReference Toplak, Ziegler, Keller, Hamann, Godin and Wittert24 have shown that a weight loss of >5% at 3 months is an accurate predictor of sustained improvements in weight in the long term.
Cardiovascular risk factors
Other than weight loss, orlistat has demonstrated beneficial effects on cardiovascular risk factors associated with obesity. Reduction on triglycerides and low-density lipoprotein (LDL) cholesterol detected on orlistat-treated patients is 10–12% higher than expected of the weight reduction. This fact is explained by the 25% reduction on the intestinal cholesterol absorption induced by the drugReference Mittendorfer, Ostlund, Patterson and Klein25.
Diminution on intestinal lipid flux has been related to a decrease in the intra-abdominal fat content, 44% over the observed with a comparable weight reduction induced by diet aloneReference Tiikkainen, Bergholm, Rissanen, Aro, Salminen and Tamminen26, Reference Torgerson, Hauptman, Boldrin and Sjostrom27.
Other beneficial effects secondary to the weight reduction induced by orlistat include the C-reactive protein, postprandial lipaemia, atherogenic lipoproteins and adipokines (interleukin-6 and α-tumour necrosis factor) reduction as well as adiponectin augmentReference Hsieh, Wang, Liu, Tung, Chien and Chen28, Reference Sutera, Marmiera, Veya-Linderb, Hänselerc, Lentzb and Vettera29. The degree of these findings and their clinical impact is yet to be evaluated. Otherwise, reduction on lipid content in organs such as the liver, improving steatosis, has also been demonstrated after treatment with orlistatReference Zelber-Sagi, Kessler, Brazowsky, Webb, Lurie and Santo30.
Type 2 diabetes mellitus
Weight loss on diabetic patients treated with orlistat is lower than that obtained in non-diabetic patients. Significant reductions in fasting glucose, glycosylated haemoglobin, total and LDL cholesterol and triglycerides are observedReference Norris, Zhang, Avenell, Gregg, Schmid and Kim31.
Beneficial effect on glycaemic control is better than that expected by weight reduction itself. Two theories try to explain this fact based on a probable improvement on insulin sensitivity induced by orlistatReference Kelley, Kuller, McKolanis, Harper, Mancino and Kalhan32, by decreasing the lipid content in insulin-sensitive tissues (as liver and muscle) or increasing glucagon-like peptide 1 levels, as an incretin-like actionReference Damci, Yalin, Balci, Osar, Korugan and Ozyazer33.
Long-term safety and efficacy of orlistat use have been proved on the randomised, placebo-controlled trial XENDOS (XENical in the prevention of Diabetes in Obese Subjects)Reference Toplak, Ziegler, Keller, Hamann, Godin and Wittert24 where 3305 obese patients were randomised to lifestyle changes plus either orlistat or placebo, for 4 years. The cumulative incidence of diabetes was 9.0% with placebo and 6.2% with orlistat, corresponding to a risk reduction of 37.3% (P = 0.0032). Treatment with orlistat resulted in significant improvements in cardiovascular risk factors that were sustained throughout the study, including blood pressure, waist circumference and lipids.
Adolescents
Different clinical trials have shown orlistat to be a safe, tolerable and effective therapeutic option in obese adolescentsReference McDuffie, Calis, Uwaifo, Sebring, Fallon and Hubbard34, Reference Chanoine, Hampl, Jensen, Boldrin and Hauptman35. Due to these characteristics, orlistat has recently been approved by the FDA for the treatment of obesity of children and teens from 12 to 16 years old.
Side-effects
The adverse effects are almost entirely related to the intestinal tract. Although the adverse events are not serious, they can be embarrassing; orlistat can be associated with increased defecation, fatty/oily stools, leaking of oil from the rectum, faecal urgency and faecal incontinence. Described side-effects were transient during the first treatment weeks and normally do not lead to drug discontinuation.
Orlistat can decrease fat-soluble vitamins, but remained within its reference rangeReference Toplak, Ziegler, Keller, Hamann, Godin and Wittert24. This risk is mitigated by an adequate diet or, exceptionally, by the daily intake of a multiple vitamin.
Rimonabant
The endocannabinoid system acts as a neuromodulator that takes part in many physiological processes, including energy balance regulation and adipocyte secretion. Central and peripheral mechanisms are involved, whose action is mediated through two identified cannabinoid (CB) receptor subtypes, CB1 and CB2.
The CB1 receptor is located in the brain, adipose tissue, lung, intestine, muscle and liver as well as in the sympathetic nodes, heart and urinary bladder. So, they are widespread all over the organism. CB2 receptors are expressed in the immune system and they do not seem to be related to energetic metabolismReference DiMarzo and Matias36. The endocannabinoid system is usually over-expressed in obese subjects and responds to external stimuli as physical activity and food intake.
Rimonabant is a selective CB1 receptor blocker, able to disrupt central and peripheral endocannabinoid tone that determines reduction of food intake and regulation of adipocyte secretion. This effect takes part in the central nervous system as well as in peripheral terminals as gastrointestinal tract and muscle tissue (Table 2). Weight reduction is the final clinical effect.
Table 2 Central and peripheral results of CB1 blockade in regulation of food intake and peripheral metabolism
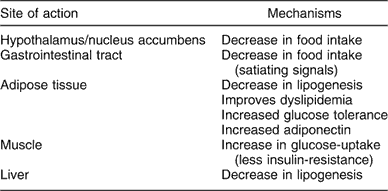
CB1 – cannabinoid receptor subtype 1.
Clinical trials
The Rimonabant-in-Obesity (RIO) programme consists of four 1–2-year phase III trials, RIO-Europe, RIO-Lipids, RIO-North America and RIO-Diabetes, designed to assess the efficacy and safety of rimonabant in the treatment of multiple cardiometabolic risk factors in 6600 overweight/obesity patients. Assignment of medication was similar in all RIO trials. Subjects were randomised to receive rimonabant 20 mg day−1, rimonabant 5 mg day−1 or placebo for 1 year.
The first published clinical trial, named RIO-EuropeReference VanGaal, Rissanen, Scheen, Ziegler and Rössner37, enrolled obese subjects with BMI >30 kg m−2, or >27 kg m−2 with a comorbidity, defined as hypertension or dyslipidaemia. One year later, significant weight reduction was observed: 67% of those who completed the trial lost 5% of the initial weight and 49% lost 10%. In addition, different components of the metabolic syndrome improved, including decrease in glycaemic and insulinaemic levels and healthier lipid profiles (reduction on triglycerides and increase in HDL cholesterol) compared to the placebo-treated group.
A second study, RIO-LipidsReference Despres, Golay and Sjostrom38, was performed in 1036 obese or overweight untreated dyslipidaemic. Results of this study showed improvement on the lipid profile as well as weight reduction (3.1 kg in the rimonabant 5 mg group and 6.9 kg in the rimobabant 20 mg group) and waist circumference decrease (7.1 cm in the rimonabant 20 mg group). HDL cholesterol increased by 23.4%, and triglycerides diminished by 15.8% in the 20 mg rimonabant group. Improvements in HDL-C and triglycerides levels with rimonabant over 1 year, compared with body weight, were beyond that attributable to weight loss alone. The C-reactive protein levels were lower in the high-dose rimonabant group (27% reduction vs. 11% for placebo, P = 0.007). Rimonabant 20 mg also increased adiponectin levels by 57.7%, a change that was partly independent of weight loss alone.
Metabolic syndrome prevalence changed from 52.9% in the beginning of the study to 25.8% after follow up on the 20 mg rimonabant group.
The RIO-Diabetes39 study, which enrolled 1047 type 2 diabetes mellitus treated on monotherapy, was designed to explore effects of rimonabant on glucose metabolism. At the end of 1 year, therapy with rimonabant 20 mg was associated with an average weight loss of 5.3 kg, compared with 1.4 kg in the placebo group. Glycosylated haemoglobin levels were decreased by 0.6% in the rimonabant 20 mg group from a baseline level of 7.3%, but were increased in the placebo group by 0.1%. Remarkably, 43% of all subjects treated with rimonabant achieved an optimal glycosylated haemoglobin level of <6.5%, compared with just 21% of those receiving placebo.
The RIO-North AmericaReference Pi-Sunyer, Aronne, Heshmati, Devin and Rosenstock40 study enrolled 3045 obese patients. The aim of the study was to evaluate whether weight loss achieved with rimonabant could be maintained after withdrawal of the drug. After 1 year of randomised design as in the other RIO trials, subjects in the two rimonabant groups underwent a second randomisation, either to continue receiving their previously assigned dose of rimonabant or to be switched to a matching placebo. Weight loss after the first year was similar to previous RIO trials (−6.3 kg for 20 mg day−1). After 2 years, subjects who followed treated with rimonabant 20 mg day−1 lost an average of 7.4 kg, whereas those subjects re-randomised to placebo had regained much of their weight. The prevalence of the metabolic syndrome declined with rimonabant 20 mg day−1 (34.8–21.1%) compared with placebo (31.7–29.2%). Lastly, the observed effects at 1 year in levels of HDL cholesterol, triglycerides, fasting insulin and in HOMA-IR (homeostatic model assessment-insulin resistance) in patients receiving 20 mg of rimonabant were approximately twice that attributable to concomitant weight loss alone.
The ongoing STRADIVARIUS trial (Strategy To Reduce Atherosclerosis Development InVolving Administration of Rimonabant – the Intravascular Ultrasound Study)41 will determine if rimonabant 20 mg day−1 administered during 18–20 months could reduce the progression of coronary atherosclerosis, as assessed by intravascular ultrasound (IVUS), when administered in patients with abdominal obesity associated with current smoking and/or metabolic syndrome, and in whom a clinically indicated coronary angiography reveals a 20–50% stenosis.
Safety and tolerability
Rimonabant treatment was well tolerated. The most common adverse effects were mild nausea and diarrhoea, probably related to gastrointestinal CB1 receptors blockade. Depressed mood, anxiety, agitation and sleep disorders were the adverse events involved in some cases of drug withdrawal.
The analysis of the published bibliography shows that rimonabant (20 mg day−1) appears to be a good new choice in the treatment of obesity and metabolic syndrome with a dual mechanism: reducing weight and improving metabolic comorbidities of obesity and possibly improving the cardiometabolic profile of obese patients.
Concluding remarks
As in other chronic illnesses, certain resistance on life-lasting treatment is at hand, firstly due to the stigmatisation of considering obesity as a sickness related to unhealthy habits and secondly because in most of the countries this kind of treatment is not financed by the National Health System; hence they become unreachable to most patients with low economical status, where obesity is more prevalent.
To date, available drugs for the treatment of obesity are safe and effective in allowing a decrease in body weight in a moderate range (5–10% initial body weight) for years, with additional properties to control associated cardiometabolic risks. However, these benefits appear only in patients who undergo long-term treatments. These questions are a matter of discussion, because in most controlled clinical trials, roughly half of the patients withdraw due to lack of efficacy and/or presence of side-effects. This situation could be partially explained by the fact that intensive lifestyle changes were not achieved in most of these studies. Greater treatment adherence and more advantageous results could be accomplished if effective behaviour modification is acquiredReference Wadden, Berkowitz, Womble, Sarwer, Phelan and Cato42. Another bias present in these clinical trials concerns the exclusion of patients on the risk of developing side-effects. Presence of hypertension, anxiety or other mood disorders is quite common in obese patients, representing a handicap on sibutramin or rimonabant use in clinical practice.
In short, the lack of financial coverage, potential contraindications, the presence of certain adverse effects and the absence of expected efficacy in some cases result in a large number of patients expecting the benefits of long-term pharmacological treatment.
Acknowledgements
Conflict of interest declaration: None.
Authorship responsibilities: All co-authors contributed equally in writing of this paper.




