Ageing and changes in body composition
It is well established that as we age we lose muscle and gain fat but an appreciation of the extent of the problems caused by loss of skeletal muscle have only recently become apparent. Age-related loss of muscle mass is gradual, starting as early as age 30 years, with increasingly greater rates of loss of approximately 1–2 %/year from the age of 50 years; see Fig. 1 ( Reference Baumgartner, Waters and Gallagher 1 – Reference Fielding, Vellas and Evans 3 ). Nutrition is integral to muscle metabolism and the present paper examines the relationship between nutrition and the chronic loss of muscle mass with age. The focus of the present paper is on chronic, not acute loss of muscle mass (as in cachexia) as the mechanisms may differ. Compared with sarcopenia cachexia is associated with medical conditions such as sepsis, cancer and immunodeficiency disease, and food intake and appetite are decreased( Reference Evans 4 – Reference Chopard, Hillock and Jasmin 10 ). Also, the signalling pathways may differ; for instance, although inflammation and cortisol play a role in development of skeletal muscle loss and sarcopenia, in cachexia, inflammatory cytokines and cortisol secretion are very greatly increased( Reference Evans 4 – Reference Chopard, Hillock and Jasmin 10 ). Additionally, in sarcopenia body weight remains relatively stable (with gradual loss of skeletal muscle), whereas in cachexia there is rapid loss of body weight and of both muscle and fat tissue which is one of the major defining factors for cachexia (weight loss >5 %)( Reference Evans 4 – Reference Chopard, Hillock and Jasmin 10 ).
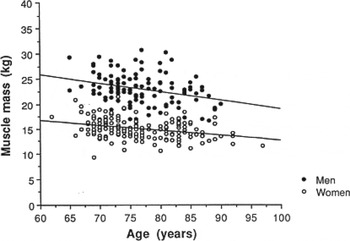
Fig. 1. Association between muscle mass and age in men and women aged 60–97 years( Reference Baumgartner, Waters and Gallagher 1 ).
Although the loss of skeletal muscle has a number of consequences, the causes are not well understood. The main nutrition focus to date has been on protein and its central role as a preventative and treatment option is not in doubt. However, other aspects of nutrition have been less well investigated although they could play an important role in terms of prevention of age-related muscle loss in middle- and older-aged adults( Reference Robinson, Cooper and Aihie Sayer 11 – Reference Rennie 23 ).
The age profile of western populations is increasing. Within the UK, there is an expected doubling of the current number of over 65 year olds (10 million) to 19 million in 2050. Additionally, of those 19 million, 6 million will be over the age of 80 years by 2050( 24 ). Worldwide, estimates suggest that 22 % of the population will be over the age of 65 years by the year 2050( Reference Beard, Biggs and Bloom 25 ). These projected increases in the age profile of populations means that the importance of the consequences and costs associated with age-related muscle loss and identifying preventative strategies will be crucial.
Consequences of age-related skeletal muscle loss
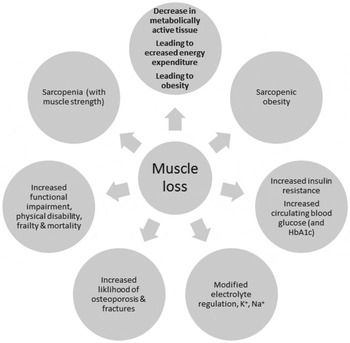
The consequences of the loss of muscle mass are the gradual loss of metabolically active tissue, accompanied by a decline in energy expenditure and loss of physical function leading to frailty and disability( Reference Beenakker, Ling and Meskers 26 – Reference Berger and Doherty 29 ). Ageing is also associated with loss of bone density leading to increases in fracture risk and to increases in insulin resistance and control of blood glucose, and with greater disturbances in electrolyte control, in all of which loss of skeletal muscle may play a role. The consequences of loss of skeletal muscle mass are summarised in Fig. 2.
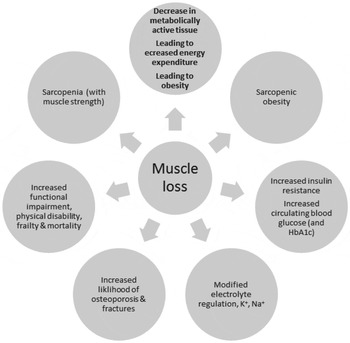
Fig. 2. The metabolic, physiological and functional consequences of the loss of skeletal muscle.
The loss of skeletal muscle mass includes changes to the type and size of muscle; with decreases in the number of muscle fibres (hypoplasia) and their size (atrophy)( Reference Narici and Maffulli 30 , Reference Faulkner, Larkin and Claflin 31 ). These changes are accompanied by a greater loss of type II than type I muscle fibres (type II, glycolytic, fast-twitch, anaerobic, used for short bursts of speed and power, being the first involved in preventing a fall). The type I fibres are oxidative, slow-twitch and aerobic. Muscle strength also declines with age (Fig. 3) and is associated with muscle loss( Reference Fielding, Vellas and Evans 3 , Reference Narici and Maffulli 30 , Reference Baumgartner, Koehler and Gallagher 32 – Reference Reid, Naumova and Carabello 34 ). Muscle mass and strength are also correlated with each other (see Fig. 4) although recent research has found that correlations are lower than found previously. In a recent study, the correlations between appendicular lean mass and muscle strength were 0·365 (P < 0·001) in men and women( Reference Fielding, Vellas and Evans 3 , Reference Narici and Maffulli 30 , Reference Baumgartner, Koehler and Gallagher 32 – Reference Chen, Nelson and Zhao 35 ). However, the decline in strength with age is proportionally greater than the decline in muscle mass( Reference Stenholm, Harris and Rantanen 36 ). Loss of muscle mass is also inversely associated with lower extremity performance in men and women( Reference Visser, Newman and Nevitt 37 ).
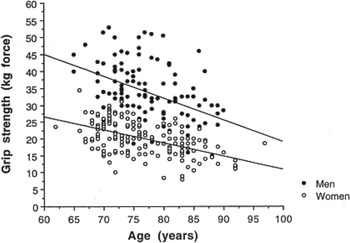
Fig. 3. Association between grip strength and age in men and women aged 60–97 years( Reference Nair 2 ).
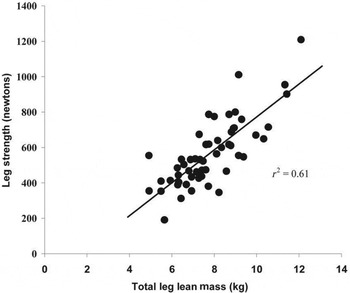
Fig. 4. Correlation between leg strength and muscle mass in men and women aged 66–84 years( Reference Reid, Naumova and Carabello 34 ).
Metabolic activity of muscle and impact on energy expenditure
Muscle plays a central role in whole-body protein metabolism and provides a reservoir of amino acids that are precursors for the synthesis of proteins as well as for hepatic gluconeogenesis, which ensures that plasma glucose concentrations are maintained( Reference Wolfe 38 ).
The consequences of the loss of muscle mass on energy expenditure occur because it is more metabolically active than adipose tissue and is responsible for about 30 % of energy expenditure( Reference Nair 2 , Reference Wolfe 38 ). The energy released as a result of muscle protein synthesis may range from ∼2029·24 kJ/d in a young man with a high proportion to muscle to ∼502.08 kJ/d in an active elderly woman( Reference Nair 2 ). These differences in energy expenditure, resulting from protein turnover, could lead to substantial differences in energy balance (providing food intake and physical activity remained constant)( Reference Nair 2 ). Wolfe calculated that the equivalent of a 10-kg difference in lean mass would lead to differences in energy expenditure that equate to a difference of 418·4 kJ/d( Reference Wolfe 38 ) and that over the course of a year this would lead to a potential gain of approximately 4·7 kg fat mass (assuming 1 kg fat stores 32216·8 kJ)( Reference Wolfe 38 ). Therefore, the loss of muscle mass could contribute to the onset of obesity via reductions in energy expenditure.
Blood glucose control and insulin resistance
Skeletal muscle accounts for ∼75 % of whole-body insulin-stimulated glucose uptake and therefore differences in the insulin resistance of muscle can affect glucose metabolism. Loss of total muscle mass also potentially influences insulin sensitivity and glucose metabolism( Reference Corcoran, Lamon-Fava and Fielding 39 ). Additionally, whole-body glucose uptake and muscle glucose transport are positively associated with type I fibres( Reference Corcoran, Lamon-Fava and Fielding 39 ). Obese people tend to have fewer type I fibres and a higher percentage of type II fibres than lean subjects( Reference Corcoran, Lamon-Fava and Fielding 39 ), and a recent comparison of different measures of muscle mass indicated that relative muscle mass relates to insulin resistance; greater muscle mass is associated with better insulin resistance( Reference Bijlsma, Meskers and van Heemst 40 ). Sarcopenia, independent of obesity, has also been associated with increased insulin resistance, in the US National Health and Nutrition Examination Survey study( Reference Srikanthan, Hevener and Karlamangla 41 ).
Regulation of extracellular potassium
Skeletal muscle is the main intracellular store of K+ and maintenance of extracellular concentrations of K are achieved through kidney excretion of K+ ( Reference McDonough, Thompson and Youn 42 ). Excretion matches to intakes and to the K+ stored in skeletal muscle( Reference McDonough, Thompson and Youn 42 ). The Na+–K+–ATPase enzyme (also known as the sodium pump/Na+–K+ pump) is located in the sarcolemma and t tubules and maintains the ion gradients that are important for muscle function and activity( Reference Clausen 43 , Reference Haddy, Vanhoutte and Feletou 44 ). The pump is responsible for K+ reuptake in skeletal muscle and maintains ion distribution by via trans-membrane gradients for Na+ and K+, and so muscle membrane potential and muscle excitability( Reference Kristensen and Juel 45 ).
With the decrease in lean body mass and increase in body fat associated with ageing there is a decrease in total body water( Reference Miller 46 , Reference Schlanger, Bailey and Sands 47 ) that occurs through reductions in the intracellular fluid compartments. Total body water reduces with age from 60 % of body weight in younger men to 54 % in men over the age of 65 years, and from 52 to 40 % in women( Reference Miller 46 , Reference Norris, Lundy and Shock 48 , Reference Watson, Watson and Batt 49 ). This change in total body water plays a role in the development of both hyponatraemia and hypernatraemia, which are more prevalent in older people( Reference Miller 46 , Reference Schlanger, Bailey and Sands 47 , Reference Luckey and Parsa 50 ). Also in a study of the muscle content of K+, Na+, Mg2+ and Ca2+, those with potassium depletion had higher concentrations of Na+ and Ca2+ in muscle and lower levels of Mg2+ than their replete counterparts( Reference Schlanger, Bailey and Sands 47 , Reference Tavichakorntrakool, Prasongwattana and Sriboonlue 51 ).
Muscle, bone density and osteoporosis
Muscle mass is positively associated with bone density, potentially due to its direct mechanical forces on bones, and so may be important in preventing falls and therefore, fractures( Reference Bogl, Latvala and Kaprio 52 – Reference Lu, Nayeem and Anderson 60 ). In addition to its role in maintaining balance, muscle mass may also act as a protective barrier to reduce the impact of falls( Reference Bogl, Latvala and Kaprio 52 – Reference Szulc, Beck and Marchand 56 ). Recently, lower muscle strength as well as muscle mass have been associated with poor cortical and trabecular bone microarchitecture( Reference Szulc, Blaizot and Boutroy 58 ).
Sarcopenia
Sarcopenia has recently been defined as the age-related loss of muscle mass and strength associated with ageing( Reference Cruz-Jentoft, Baeyens and Bauer 13 , Reference Cooper, Dere and Evans 61 ). Sarcopenia and loss of muscle mass is also associated with increased rates of mortality in older age and to functional impairment, physical disability and frailty( Reference Janssen, Heymsfield and Ross 62 – Reference Broadwin, Goodman-Gruen and Slymen 65 ).
Estimated rates of prevalence of sarcopenia range from 9 to 18 % in those over the age of 65 years to up to 50 % in those over 80 years old( Reference Fielding, Vellas and Evans 3 , Reference Sayer 66 , Reference Abellan van Kan 67 ). The current costs of sarcopenia in the USA are estimated as $18·5 billion ($10·8 billion in men and $7·8 billion in women), about 1·5 % of total health care expenditure( Reference Sayer 66 , Reference Janssen, Shepard and Katzmarzyk 68 ).
In addition to the direct estimated costs of sarcopenia, sarcopenia contributes to fractures (through the impact on falls) which are a major public health problem, costing £2·3 billion per year in health and social care in the UK alone and $17 billion per year in the USA( Reference Harvey, Dennison and Cooper 69 , 70 ).
Sarcopenic obesity can also exist (high fat mass and low muscle mass) because the reduction in muscle mass associated with ageing can be accompanied by accumulation of fat mass( Reference Silva, Karnikowski and Funghetto 71 – Reference Baumgartner 73 ). Estimates of the prevalence of sarcopenic obesity are between 34 and 48 % in the elderly although these estimates depend on the method of definition( Reference Silva, Karnikowski and Funghetto 71 , Reference Batsis, Barre and Mackenzie 74 ). A recent study in about 5000 men and women from the National Health and Nutrition Examination Survey study found estimates of sarcopenic obesity varied between 4·4–84 % in men, and 3·6–94 % in women and recommended that methods of estimating prevalence of sarcopenia needed to be more consistent( Reference Batsis, Barre and Mackenzie 74 ). Obesity is a risk factor for poor health, reduced functional capacity and quality of life, as is loss of muscle mass and strength. Sarcopenic obesity combines the effects and mechanisms of both sarcopenia and obesity, potentially exacerbating both in older people( Reference Rolland, Lauwers-Cances and Cristini 28 , Reference Stenholm, Harris and Rantanen 36 , Reference Batsis, Barre and Mackenzie 74 – Reference Li and Heber 78 ). Both sarcopenia and obesity are associated with inflammation and increased insulin resistance and these mechanisms may act synergistically to worsen both obesity and muscle loss in overweight elderly people( Reference Stenholm, Harris and Rantanen 36 , Reference Batsis, Barre and Mackenzie 74 – Reference Roubenoff 77 ). Thus being overweight and having lower muscle mass and strength is not only a risk factor for reduced functional capacity but may also act in a vicious circle to potentiate and worsen the situation, with ever increasing fat mass and lower muscle mass in those with sarcopenic obesity( Reference Stenholm, Harris and Rantanen 36 , Reference Batsis, Barre and Mackenzie 74 – Reference Roubenoff 77 ).
Maintenance of muscle mass is therefore highly important for maintaining metabolic health, for potentially preventing the onset of insulin resistance and obesity and osteoporosis and for prevention of the onset of frailty and sarcopenia and their consequences on fractures and on morbidity and mortality.
Measurement of muscle mass
Muscle mass can be assessed in a number of ways ranging from anthropometric circumference methods to MRI scanning. Dual energy X-ray absorptiometry (DXA) is considered the most accurate, reliable and least expensive method for research( Reference Cooper, Dere and Evans 61 , Reference Abellan van Kan 67 ). From this, appendicular skeletal muscle mass is calculated which is the sum of muscle mass of the four limbs (measured using DXA). Appendicular skeletal muscle is used to calculate skeletal muscle mass index: appendicular skeletal muscle/height2 (height2 is used to account for the proportional increase in muscle mass with increase in height)( Reference Kyle, Genton and Gremion 79 ). This index and the fat-free mass (FFM) index (FFM/height2) are frequently used in population research for loss of muscle mass and for diagnosis of sarcopenia( Reference Fielding, Vellas and Evans 3 , Reference Baumgartner, Koehler and Gallagher 32 , Reference Abellan van Kan 67 , Reference Bijlsma, Meskers and Ling 80 ). Although not widely reported, total FFM and appendicular lean mass are highly correlated in women 0·97–0·99 (P < 0·001)( Reference Welch, MacGregor and Minnihane 81 , Reference Schautz, Later and Heller 82 ) and 0·98 in men( Reference Schautz, Later and Heller 82 ). Other indices may also be used but comparison studies show they provide different estimates of sarcopenia( Reference Fielding, Vellas and Evans 3 , Reference Baumgartner, Koehler and Gallagher 32 , Reference Abellan van Kan 67 , Reference Bijlsma, Meskers and Ling 80 ). Measurement of thigh and mid-upper arm circumferences is inexpensive and simple but suffers from measurement error. Bioelectrical impedance is more convenient but there is a loss of accuracy and reliability with this method. The specificity of calf circumference using a specific cut-point (<31 cm) was found to have only 44·3 % sensitivity( Reference Visser 83 ). Computerised tomography and MRI scans are both useful tools for research but are expensive, with computerised tomography scans involving greater radiation exposure than DXA( Reference Abellan van Kan 67 ). Urinary 24-h creatinine excretion directly measures muscle mass, and changes in excretion of total nitrogen can indicate differences in muscle turnover (as nitrogen retention or loss)( Reference Heymsfield, Arteaga and McManus 84 – Reference Frassetto, Morris and Sebastian 86 ). An indirect method for measurement of skeletal muscle is whole-body 40K derived total potassium( Reference Gallagher, Visser and De Meersman 87 ). Very recently a method for measuring total body protein has been developed by using a model based on DXA and impedance measurements and this has been compared with neutron activation analysis (a criterion measure for in vivo measures of total body protein)( Reference Wilson, Strauss and Fan 88 ).
Overview of mechanisms associated with nutrition and muscle loss
Loss of muscle is due to the effects on protein turnover, i.e. the imbalance between protein anabolism and catabolism. There are a number of recent reviews which detail mechanisms associated with muscle loss to which the reader can refer( Reference Fielding, Vellas and Evans 3 , Reference Chopard, Hillock and Jasmin 10 , Reference Narici and Maffulli 30 , Reference Bonaldo and Sandri 89 – Reference Schiaffino, Dyar and Ciciliot 92 ). In terms of muscle anabolism, protein as amino acids is essential for muscle( Reference Mallinson and Murton 93 ). The mechanism that controls entry of branched chain amino acids into metabolic pathways is the mTOR pathway (originally ‘mammalian TOR’, now referred to as ‘mechanistic TOR’)( Reference Rennie, Wackerhage and Spangenburg 91 , Reference Mallinson and Murton 93 – Reference Russell 95 ). mTOR is a kinase downstream of insulin and nutrient-sensing pathways that is required for cell growth( Reference Bonaldo and Sandri 89 ). Insulin-like growth factor (IGF-1) signalling induces anabolism, being up-regulated during resistance exercise, and this influences the IGF-1–Akt–FoxO pathway( Reference Bonaldo and Sandri 89 , Reference Banerjee, Apponi and Pavlath 96 , Reference Perrini, Laviola and Carreira 97 ). This pathway induces skeletal muscle hypertrophy by increasing protein synthesis as well as also blocking protein degradation( Reference Banerjee, Apponi and Pavlath 96 , Reference Banerjee and Guttridge 98 ). A progressive age-related decline in de novo synthesis of mixed muscle protein, myosin heavy chain and mitochondrial protein has been demonstrated though the reasons for this have yet to be elucidated( Reference Nair 2 , Reference McCoy and Nair 90 ). However, there is also potential anabolic resistance with ageing( Reference Robinson, Cooper and Aihie Sayer 11 , Reference Millward 99 ). Aged muscle is characterised by a defect in the ability of leucine to stimulate protein synthesis( Reference Fielding, Vellas and Evans 3 , Reference Lang, Streeper and Cawthon 59 , Reference Mosoni, Balage and Vazeille 100 ). Satellite cells may control myogenesis and are a population of undifferentiated myogenic cells that supply myoblasts for growth, homoeostasis and repair( Reference Bonaldo and Sandri 89 , Reference Scharner and Zammit 101 , Reference Snijders, Verdijk and van Loon 102 ). They are located between the basal lamina and plasma membrane of muscle fibres( Reference Bonaldo and Sandri 89 , Reference Scharner and Zammit 101 , Reference Snijders, Verdijk and van Loon 102 ).
In terms of catabolism, the ubiquitin proteasome system is responsible for the removal of damaged proteins after muscle activity. Other processes that remove damage to cells and remove damaged cells are apoptosis (programmed cell death) and autophagy (mechanism responsible for degradation of cytoplasmic contents) with macro autophagy and chaperone-mediated autophagy thought to be potentially important( Reference Narici and Maffulli 30 , Reference Banerjee and Guttridge 98 ). It is thought that the proper balance of the autophagic flux is essential for maintaining healthy skeletal muscle( Reference Bonaldo and Sandri 89 ). Other processes found to influence catabolism and muscle loss in human or animal models are reductions in hormones with age (testosterone, oestrogen and IGF-1), insulin resistance, mitochondrial ageing, oxidation and inflammation( Reference Peterson, Johannsen and Ravussin 103 ).
The signalling pathways that regulate the size of myofibres and the contractile performance of muscle crosstalk and modulate one another at different levels, coordinating protein synthesis and degradation simultaneously( Reference Bonaldo and Sandri 89 ). Four of the major signalling pathways are the IGF-1–Akt–FoxO pathway, myostatin, NFκB and glucocorticoids( Reference Fielding, Vellas and Evans 3 , Reference Bonaldo and Sandri 89 ). In the IGF-1–Akt–FoxO pathway, Akt controls protein synthesis via mTOR, and protein degradation via transcription factors of the FoxO family( Reference Bonaldo and Sandri 89 ). FoxO also signals between protein breakdown and synthesis, with FoxO3 playing a role in suppression of protein synthesis, and Akt plays a role in anabolism by supressing protein breakdown( Reference Bonaldo and Sandri 89 ). FoxO are major regulators of the ubiquitin proteasome system acting by directly regulating muscle-specific E3 ligases( Reference Banerjee and Guttridge 98 ). Inflammatory cytokines (C-reactive protein, TNF-α) have a potential role to play in muscle loss with the effect of inflammatory cytokines mediated by the NFκB transcription factors, which are expressed in skeletal muscle( Reference Bonaldo and Sandri 89 , Reference Nicastro, da Luz and Chaves 104 ). A high inflammatory cytokine status has been found to be associated with loss of muscle mass and strength in older people aged 70–79 years at baseline( Reference Cooper, Dere and Evans 61 , Reference Schaap, Pluijm and Deeg 105 ).
5′-AMP-activated protein kinase is involved in the regulation of muscle size and may exert effects through both protein synthesis and degradation( Reference Goodman, Mayhew and Hornberger 106 ). It is a ‘fuel-sensing’ enzyme present in all mammalian cells( Reference Goodman, Mayhew and Hornberger 106 ). Myostatin is expressed in skeletal muscle and is a member of the transforming growth factor-β family that acts to inhibit muscle growth( Reference Bonaldo and Sandri 89 , Reference Banerjee and Guttridge 98 ). Although the mechanisms whereby myostatin influences muscle loss are not completely understood, it is thought that myostatin may inhibit the Akt pathway. Increased glucocorticoid levels are associated with muscle loss, acting by inhibiting amino acid transport into muscle and by increasing muscle catabolism by activating the IGF-1–Akt–FoxO and NFκB pathways( Reference Bonaldo and Sandri 89 , Reference Hanaoka, Peterson and Crofford 107 ). Insulin is the major hormone responsible for modifying protein degradation (via the Akt–FoxO pathway), and insulin resistance can result in increased degradation of skeletal muscle( Reference Honors and Kinzig 6 , Reference Guillet and Boirie 108 ). Of the mechanisms cited here, the responsiveness of 5′-AMP-activated protein kinase activation declines during ageing, and ageing is also associated with increases in glucocorticoid secretion and insulin resistance, so there is an interaction between these mechanisms and ageing( Reference Michalakis, Goulis and Vazaiou 109 – Reference Bremer, Mietus-Snyder and Lustig 112 ). However, the evidence for an increase in myostatin levels in blood with ageing is equivocal( Reference Sakuma and Yamaguchi 113 , Reference Han and Mitch 114 ).
Oxidative stress and the accumulation of reactive oxygen species (ROS), generated during oxidative metabolism, potentially contribute to age-related muscle loss( Reference Liochev 115 ). Skeletal muscle is the highest consumer of oxygen in the body due to its high requirements, and the high proportion of skeletal muscle, so there is potential for high levels of ROS to be produced within (the mitochondria of) skeletal muscle( Reference Doria, Buonocore and Focarelli 116 ). Oxidative stress may trigger the imbalance between protein synthesis and degradation in muscle although the exact mechanism is, as yet, unknown( Reference Doria, Buonocore and Focarelli 116 ). Within mitochondria, oxidative stress can induce higher rates of cellular damage to DNA, proteins and membranes as well as the associated metabolic functions of mitochondria, and markers of oxidative damage to DNA, proteins and lipids are elevated in skeletal muscle of older adults( Reference Doria, Buonocore and Focarelli 116 , Reference Semba, Lauretani and Ferrucci 117 ). Oxidative stress may also induce loss of viability of satellite cells leading to their shorter lifespan and a decrease in their proliferative capacity, and may also impact on myofibril structure and the functional status of calcium channels responsible for muscle contraction( Reference Kim, Wilson and Lee 118 ). While ROS have been found to build up in the mitochondria of the muscle of older rats and human subjects, potentially attenuating signalling arising from mitochondria, more recent research has identified that redox signalling (ROS stimulation of intra and extracellular messengers) is relevant for the signalling pathways involved in force production during muscle contraction( Reference Jackson 119 ). Redox signalling is also involved in glucose uptake and in insulin signalling( Reference Jackson 119 ). These non-mitochondrial redox signalling pathways, initiated by ROS for muscle contraction, have been found to be ‘abolished’ in old age( Reference Jackson 119 ).
Biological and behavioural factors affecting loss of muscle mass
The biological and behavioural factors that affect loss of skeletal muscle mass are summarised in Table 1. Age is well established as a major cause of muscle loss and this may be related to changes in hormonal status( Reference Cooper, Dere and Evans 61 , Reference Baumgartner 73 , Reference Messier, Rabasa-Lhoret and Barbat-Artigas 120 ). In women, the decline in oestrogen levels around the menopause may play a role, although evidence relating hormonal status and muscle loss is not consistent( Reference Cooper, Dere and Evans 61 , Reference Messier, Rabasa-Lhoret and Barbat-Artigas 120 , Reference Brown 121 ). Similarly, in men testosterone status may also be related( Reference Baumgartner, Waters and Gallagher 1 , Reference Cooper, Dere and Evans 61 , Reference Baumgartner 73 , Reference Messier, Rabasa-Lhoret and Barbat-Artigas 120 , Reference Szulc, Duboeuf and Marchand 122 ).
Table 1. Summary of the biological, nutritional and lifestyle factors affecting age-related loss of skeletal muscle mass

* Established factors
† Less established factors
‡ Potential factors
§ Factors that reduce with age
Smoking is related to lower muscle mass and may operate through mechanisms that influence protein turnover( Reference Szulc, Duboeuf and Marchand 122 – Reference Newman, Kupelian and Visser 124 ). Tobacco smoke contains a complex cocktail of compounds (oxygen- and nitrogen-free radicals and ROS such as hydrogen peroxide and superoxide) that could influence protein turnover through the 5′-AMP-activated protein kinase and NFκB pathways( Reference Rom, Kaisari and Aizenbud 123 ).
Physical activity is an important influence in maintaining muscle mass and strength with interventions using resistance activity generally resulting in increased muscle mass in older adults( Reference Wolfe 38 , Reference Szulc, Duboeuf and Marchand 122 , Reference Newman, Kupelian and Visser 124 ). However, diet may be particularly relevant in sedentary individuals. One study that compared the effects of a high-fat diet with different levels of exercise, on the fatty acid profile of rat skeletal muscle, found that diet had a much greater effect than training on the fatty acid profile of oxidative and glycolytic rodent muscle( Reference Turner, Lee and Bruce 125 ).
Taller, heavier people have greater skeletal muscle mass than those who are smaller and lighter( Reference Wolfe 38 , Reference Gallagher, Visser and De Meersman 87 , Reference Newman, Kupelian and Visser 124 , Reference Estrada, Kleppinger and Judge 126 , Reference Koster, Ding and Stenholm 127 ). However, although longitudinal studies found that baseline lean mass is greater in those with more fat mass, over time more lean mass is lost in those with greater fat mass (over 7 years)( Reference Koster, Ding and Stenholm 127 ).
Weight loss and ‘yo yo’ dieting or cyclical change in body weight’ may be a risk factor for sarcopenia as this results in reduction in both lean and fat mass and may, over time, result in net loss of lean mass( Reference Prentice, Jebb and Goldberg 128 – Reference Steigler and Cunliffe 130 ). The majority of carefully controlled intervention studies have not found evidence for greater than expected loss of lean mass with cyclical change in body weight, in human or animal studies or with long-term energy restriction( Reference Prentice, Jebb and Goldberg 128 – Reference Solomon, Sistrun and Krishnan 134 ). However, recent epidemiological studies have found that even with regain of weight there is a net loss of lean mass, suggesting that weight loss may contribute to sarcopenia in older adults( Reference Newman, Lee and Visser 135 , Reference Lee, Visser and Tylavsky 136 ). The extent of loss of lean body mass may also be dependent on the macronutrient composition of the diet, with recent studies suggesting that weight loss diets containing a higher percentage protein or with a higher glycaemic index may be more protective for muscle( Reference Steigler and Cunliffe 130 , Reference Das, Gilhooly and Golden 131 , Reference Mojtahedi, Thorpe and Karampinos 137 ).
Comparatively very few studies have investigated the relationship between muscle strength or function and diet although a recent study found total energy and protein were related to grip strength and protein was also related to standing time, in middle-aged men and women over a 7-year period( Reference Robinson, Cooper and Aihie Sayer 11 , Reference Mithal, Bonjour and Boonen 138 , Reference Mulla, Cooper and Mishra 139 ). Another study found grip strength was positively related to fish intake in older-aged men and women and an intervention found fish oils enhanced strength training in men( Reference Robinson, Jameson and Batelaan 140 , Reference Rodacki, Rodacki and Pereira 141 ).
The remainder of the present paper describes established areas of known associations with nutrition and muscle loss (protein) and identifies other less investigated areas with suggestions for potential for research and prevention. The specific mechanisms by which nutrients can influence skeletal muscle loss are also detailed within the sections that follow and the nutrients and bioactive compounds associated, to date, with muscle loss are also summarised in Table 1.
Nutrition and age-related muscle loss
Protein
Protein has been the most intensively studied nutrient in relation to muscle mass in terms of absolute or percentage of energy intake or as amino acids.
Protein turnover is integral to muscle and as discussed previously, in the section on mechanisms, the balance between anabolism and catabolism is important for maintenance of skeletal muscle mass. The major metabolic pathway through which essential amino acids act to produce an anabolic response is the mTOR pathway and current opinion is that this is regulated by the branched chain amino acid leucine( Reference Dillon 142 ). Recent evidence suggests that the threshold concentration of circulating amino acids required to produce an anabolic response in skeletal muscle synthesis may be increased in older muscle, and that increasing concentrations of leucine are required to maintain robust anabolic responses( Reference Dillon 142 ). A number of recent publications have extensively reviewed the literature on interventions and mechanisms of protein and amino acid intake in relation to muscle mass, with evidence on total protein intake, type and quantity of amino acid and type of protein (casein/whey v. soya) to which the reader can refer for more detail( Reference Dillon 142 – Reference Cermak, de Groot and van Loon 145 ).
Cross-sectional studies and protein intake
Studies associating protein intake and muscle mass in population cross-sectional studies have not been previously reviewed extensively but those included here have found contradictory associations( Reference Baumgartner, Waters and Gallagher 1 , Reference Scott, Blizzard and Fell 15 , Reference Meng, Zhu and Devine 22 , Reference Mitchell, Haan and Steinberg 146 – Reference Stookey, Adair and Popkin 148 ). Protein intake was not associated with muscle mass in older men and women in two studies( Reference Baumgartner, Waters and Gallagher 1 , Reference Mitchell, Haan and Steinberg 146 ) but was positively related in another( Reference Scott, Blizzard and Fell 15 ), with a difference of 1·3 kg between quartiles 1 and 4 (P = 0·002)( Reference Scott, Blizzard and Fell 15 ); also at follow-up it was associated with change in appendicular lean mass over a two and a half-year period( Reference Scott, Blizzard and Fell 15 ). A lower percentage protein intake was associated with greater loss of mid-arm muscle area (<10·4 % protein as percentage energy compared with 10·4–12·1 % energy) over 4 years, in Chinese men and women aged 50–69 years( Reference Stookey, Adair and Popkin 148 ). In addition, in men and women aged 70–79 years, energy adjusted protein intake was associated with 3-year changes in lean mass( Reference Houston, Nicklas and Ding 147 ). Those with a higher percentage protein intake had a lower rate of loss of lean mass and appendicular lean mass, by ∼40 %, compared with the lowest( Reference Houston, Nicklas and Ding 147 ). Finally, in Australian women aged 72–78 years lean mass, appendicular lean mass and upper arm muscle area were all positively and significantly associated with baseline protein intake( Reference Meng, Zhu and Devine 22 ). Compared with the lowest tertile of protein intake (<66 g/d v. >87 g/d) there were 5·4–6·0 % differences in whole body and appendicular lean mass( Reference Meng, Zhu and Devine 22 ).
Interventions with protein and amino acids
A large number of intervention trials with protein, whey, casein and mixed essential or individual amino acids have been performed, although very few have been dietary or supplementation studies alone( Reference Malafarina, Uriz-Otano and Iniesta 144 ). Of the few studies of amino acid or protein supplementation only, in interventions with essential amino acids, two studies found increases in lean body mass over the intervention period( Reference Dillon 142 – Reference Malafarina, Uriz-Otano and Iniesta 144 , Reference Dillon, Sheffield-Moore and Paddon-Jones 149 , Reference Borsheim, Bui and Tissier 150 ). Generally, the response to protein and amino acid supplementation (with or without exercise interventions) has not been consistent and this may be due to differences in nutritional status at baseline, differing methods of measuring outcomes and geographical and racial differences( Reference Malafarina, Uriz-Otano and Iniesta 144 , Reference Casperson, Sheffield-Moore and Hewlings 151 ). Supplementation studies with protein have not always enhanced the effects of resistance training( Reference Candow, Forbes and Little 152 , Reference Kukuljan, Nowson and Sanders 153 ).
In a recent meta-analysis of twenty-two randomised controlled trials of protein supplementation studies (in the form of protein, whey, casein and mixed essential or individual amino acids) accompanied by resistance exercise interventions, protein supplementation had an overall positive effect on FFM and leg strength( Reference Cermak, Res and de Groot 154 ). Protein supplementation had a similar effect in both younger and older participants (>50 years) and also increased the gain in type II muscle fibre cross-sectional area overall, but in further analysis this was not apparent in older subjects( Reference Cermak, Res and de Groot 154 ).
The research to date from both cross-sectional and intervention studies indicates that sufficient protein of high quality is undoubtedly crucial for muscle mass, with leucine being the most effective amino acid. However, other nutritional factors may also influence muscle mass as well as protein but have been much less studied. The following sections cover these less researched areas relating nutrition to age-related muscle loss.
Vitamin D
The mechanisms that explain the relationship of vitamin D with muscle mass and strength may be direct or indirect either through calcium handling and signalling and accumulation in the sarcoplasmic reticulum (influencing the involvement of calcium in muscle contraction) or through the activation of vitamin D receptors found in muscle( Reference Ceglia and Harris 155 ). Vitamin D deficiency is associated with atrophy of type II muscle fibres( Reference Ceglia and Harris 155 ). However, in contrast to prevailing opinion and previous studies, a recent study was unable to detect vitamin D receptors in human skeletal muscle, suggesting the activity of vitamin D on muscle may be indirect( Reference Wang and DeLuca 156 ). Nevertheless, an earlier study found that vitamin D receptor polymorphisms in muscle were associated with lower FFM in older men and women( Reference Scott, Blizzard and Fell 157 ). Vitamin D status has also been associated with muscle strength in a number of studies, i.e. better vitamin D status associated with greater muscle strength( Reference Ceglia and Harris 155 ).
Comparatively few studies have assessed vitamin D status and associated it with muscle mass, although two early intervention studies found a positive effect of vitamin D supplementation on type IIa or II muscle fibres( Reference Ceglia and Harris 155 ). Four cross-sectional studies found a positive association between vitamin D status and total or appendicular muscle mass in men and women( Reference Szulc, Duboeuf and Marchand 122 , Reference Marantes, Achenbach and Atkinson 158 – Reference Visser, Deeg and Lips 160 ), although in one of the studies the association was found only in women under the age of 65 years; not in men and older women( Reference Marantes, Achenbach and Atkinson 158 ). Two of these studies, after 2·6 or 3 years follow-up, found that protection from muscle loss was associated with baseline vitamin D status( Reference Scott, Blizzard and Fell 159 , Reference Visser, Deeg and Lips 160 ). A further cross-sectional study did not find an association between vitamin D status and muscle area (using computed tomography) but did find that infiltration of fat into muscle was greater in those with lower vitamin D status (plasma≤29 ng/ml)( Reference Gilsanz, Kremer and Mo 161 ).
Although vitamin D is highly likely to influence muscle mass further research is needed to establish definitively the extent to which vitamin D can positively influence muscle mass.
Alcohol
Extreme habitual consumption of alcohol (>80 g alcohol/d) can lead to chronic alcoholic myopathy, development of muscle weakness and wasting( Reference Fernandez-Sola, Preedy and Lang 162 – Reference Preedy, Paice and Mantle 164 ). Even in healthy volunteers drinking about 22 units daily over a month induces substantial muscle damage( Reference Song and Rubin 165 ). The frequency of alcoholic myopathy is estimated as being between 40 and 60 % in those who are alcoholic( Reference Wijnia, Wielders and Lips 166 ). An acute alcoholic myopathy can also occur after substantial alcohol consumption, even in healthy people, and is accompanied by muscle aching and tenderness, and often muscle cramps( Reference Wijnia, Wielders and Lips 166 , Reference Hewitt and Winter 167 ). The extreme loss of muscle mass that occurs in alcoholic patients may be reversed by becoming abstinent, as one study found significantly increased total lean mass in alcoholics who abstained from drinking (∼600 g over 6 months)( Reference Martin-Gonzalez, Gonzalez-Reimers and Santolaria-Fernandez 168 ).
The structural damage to skeletal muscle that occurs with alcohol includes reduced diameter of type II fibres, particularly the type IIb fibres (which do not include mitochondria) whereas the type I fibres appear unaffected, and in the early stages show compensatory hypertrophy( Reference Preedy and Emery 163 , Reference Wijnia, Wielders and Lips 166 ). The extent of alcoholic myopathy is sufficiently severe to be measurable by changes to mid-arm circumference and DXA measurements( Reference Preedy and Emery 163 , Reference Martin-Gonzalez, Gonzalez-Reimers and Santolaria-Fernandez 168 ).
The mechanisms involved in alcoholic myopathy include reduced rates of protein synthesis and breakdown, loss and redistribution of ribosomal RNA, increased RNase activities, membrane damage, altered Ca2+ regulation and the generation of free radicals( Reference Preedy and Emery 163 , Reference Preedy, Paice and Mantle 164 , Reference Pacy, Preedy and Peters 169 ). Alcohol can also inhibit hepatic stimulation of IGF-1 and disrupt the growth hormone/IGF-1 axis( Reference Ronis, Wands and Badger 170 ). Secretion of glucocorticoids and inflammatory cytokines are other mechanisms underlying alcoholic myopathy( Reference Ronis, Wands and Badger 170 ).
Although extreme loss of muscle mass has been identified in alcoholics, very little population research on impact of high alcohol consumption has been done. In one study of Korean men, older than 60 years, and one in France in older men, found no association between alcohol intake and sarcopenia( Reference Szulc, Duboeuf and Marchand 122 , Reference Kim, Kim and Hwang 171 ). However, the distribution of high alcohol consumption was relatively low in the Korean population with the highest category of alcohol consumption being >7 drinks at a time >2 d/week which may explain the lack of findings in that study( Reference Kim, Kim and Hwang 171 ).
Alcohol consumption could contribute to the loss of skeletal muscle at the population level as in some population groups such as young men in the UK aged 18–24 years 40 % drink more than the recommended 21 units per week and 25 % of women more than 14 units per week( Reference Preedy and Emery 163 ). This could be a potential future problem given that alcohol use is increasing, and further research is required.
Dietary acid–base load
Metabolic acidosis is one of the major causes of skeletal muscle loss in chronic kidney disease, and the mild metabolic acidosis that occurs within populations may also be relevant for loss of skeletal muscle( Reference Mitch 172 – Reference Welch, Macgregor and Skinner 174 ).
The acid–base balance in blood is partly maintained by the balance between H+ and bicarbonate ions. Metabolic acidosis is associated with a more acidic blood acid–base composition and, while the acid–base equilibrium is maintained within narrow limits, the pH in blood becomes more acidic with age( Reference Frassetto, Morris and Sebastian 175 ). This is because maintenance of the blood acid–base system is dependent on excretion of H+ ions by the urine, and as renal function gradually declines with age there is potential for mild metabolic acidosis to increase( Reference Frassetto, Morris and Sebastian 175 ).
Diet has the potential to contribute to mild metabolic acidosis in the general population through consumption of potentially acidogenic and alkalinogenic forming foods. Potentially acidogenic foods are protein containing foods that contain the sulphur-containing amino acids (cysteine and methionine) which when metabolised lead to the production of hydrogen ions that can lower blood pH( Reference Welch, Mulligan and Bingham 176 – Reference Wynn, Krieg and Aeschlimann 180 ). The major contributing acidogenic foods are meats, fish, eggs, cereals and dairy foods and these foods have been positively associated with a more acidic urine pH in population studies( Reference Welch, Mulligan and Bingham 176 – Reference Wynn, Krieg and Aeschlimann 180 ). The alkalinogenic foods, fruits and vegetables that balance the H+ ions generated via metabolism of acidogenic foods, through the carbonate compounds present in them, can lower increase blood pH( Reference Welch, Mulligan and Bingham 176 – Reference Wynn, Krieg and Aeschlimann 180 ). The overall dietary balance between acidogenic foods (protein-containing foods) and alkalinogenic foods (fruits and vegetables), that supply base precursors, is the dietary acid–base load, and a number of population and intervention studies have demonstrated associations between diet and measures of metabolic acidosis. There are a number of methods of estimating diet-dependent net acid load and the balance between acidogenic and alkalinogenic foods and these include estimated net endogenous acid production or potential renal acid load( Reference Frassetto, Lanham-New and Macdonald 181 ). Potential renal acid load includes contributions from nutrient categories that relate to the major determinants of their acid or base-forming potential, i.e. protein and phosphorus (acidogenic) and calcium, potassium and magnesium (alkalinogenic)( Reference Welch, Mulligan and Bingham 176 – Reference Frassetto, Todd and Morris 178 ).
A number of mechanisms have been proposed for the loss of muscle mass with metabolic acidosis, largely derived from animal studies and studies on those with chronic kidney disease( Reference Mitch 172 – Reference Welch, Macgregor and Skinner 174 ). Metabolic acidosis potentially accelerates proteolysis and amino acid catabolism, probably through activation of caspase-3 and the ubiquitin proteasome system or via effects on the growth hormone/IGF-1 axis( Reference Perrini, Laviola and Carreira 97 , Reference Dawson-Hughes, Harris and Ceglia 182 – Reference Thomas and Mitch 186 ).
The relationship between supplementation with bicarbonate compounds, designed to attenuate metabolic acidosis, has been investigated in a few studies of middle- and older-aged populations and two of these studies found attenuation of nitrogen excretion, with the more recent study suggesting this might potentially be mediated by IGF-1( Reference Frassetto, Morris and Sebastian 86 , Reference Dawson-Hughes, Harris and Ceglia 182 , Reference Dawson-Hughes, Castaneda-Sceppa and Harris 187 – Reference Dawson-Hughes, Harris and Palermo 189 ). A reduction in nitrogen excretion indicates more nitrogen has been retained for the anabolic processes in muscle. Two intervention studies designed to understand the effect of reducing the metabolic acidosis of chronic renal disease, using sodium bicarbonate as the source of bicarbonate, found improvements in nitrogen balance and in serum albumin and mid-arm muscle circumference after supplementation for 2 years( Reference Papadoyannakis, Stefanidis and McGeown 190 , Reference de Brito-Ashurst, Varagunam and Raftery 191 ).
However, to date the effects of dietary metabolic acid–base load on muscle mass in healthy participants have only been investigated in two studies( Reference Welch, Macgregor and Skinner 174 , Reference Dawson-Hughes, Harris and Ceglia 182 ). In one an alkaline diet, as measured by potassium excretion was positively related to muscle mass and change in lean body mass over a 3-year period( Reference Dawson-Hughes, Harris and Ceglia 182 ). In the other, a more alkalinogenic diet was positively related to indices of muscle mass and the proportion of fruits and vegetables to alkaline-forming foods (meat, fish, eggs and dairy)( Reference Welch, Macgregor and Skinner 174 , Reference Dawson-Hughes, Harris and Ceglia 182 ). The associations remained significant after accounting for total protein intake and other confounders that influence muscle mass. The scale of the association ranged between a fifth and one-half of the observed relationship with 10 years of age, i.e. the alkalinogenic effect of diet was about half as important as the effect of age on muscle loss( Reference Frassetto, Morris and Sebastian 86 , Reference Dawson-Hughes, Harris and Ceglia 182 , Reference Dawson-Hughes, Castaneda-Sceppa and Harris 187 – Reference Dawson-Hughes, Harris and Palermo 189 ). In that study the optimal ratio of alkalinogenic to acidogenic foods was about 1·4.
These results suggest that fruits and vegetables as well as protein may be important for conservation of muscle; however, intervention studies to investigate the effects of a more alkaline diet on muscle loss in populations have yet to be done.
Interestingly, a recent rodent study designed to understand the interaction between vitamin D and alkaline status on the loss of muscle mass, found an interaction between vitamin D and alkaline status( Reference Ceglia, Rivas and Pojednic 192 ). Vitamin D status modified the effect of potassium bicarbonate supplementation (alkalinising compound) on muscle mass (after 12 weeks)( Reference Ceglia, Rivas and Pojednic 192 ). It also influenced Akt activation (Akt regulates several signalling pathways in skeletal muscle)( Reference Ceglia, Rivas and Pojednic 192 ). The data suggested that alkali supplementation was effective in increasing muscle but that this effect was more enhanced when vitamin D status was adequate( Reference Ceglia, Rivas and Pojednic 192 ). Therefore, future work in this field also needs to take into account vitamin D status.
Dietary fat composition
The association between dietary fat and muscle mass has been relatively under investigated which, given the central role of fat in muscle metabolism, is surprising. The fatty acids derived from dietary fat are the major source of energy for resting and working muscle and fats are an integral component of myocellular membranes( Reference Corcoran, Lamon-Fava and Fielding 39 , Reference Welch, MacGregor and Minnihane 81 , Reference Turner, Lee and Bruce 125 , Reference Kiens 193 – Reference Kien 204 ). Dietary fat composition also influences inflammation and insulin resistance, mechanisms recently identified as relating to potential loss of FFM( Reference Corcoran, Lamon-Fava and Fielding 39 , Reference Kiens 193 – Reference Frayn 195 , Reference Kien 204 – Reference Holloway, Luiken and Glatz 208 ).
During exercise fatty acid entry into cells is principally regulated by the proteins fatty acid translocase and fatty acid binding protein, although at high intensities of exercise the contribution of fat to oxidative metabolism declines( Reference Kiens 193 , Reference Frayn 194 , Reference Holloway, Bonen and Spriet 205 , Reference Holloway, Luiken and Glatz 208 , Reference Dyck 209 ). It is also known that fatty acids are differentially oxidised (from whole-body studies), with unsaturated fatty acids and oleic acid oxidised preferentially over SFA( Reference Corcoran, Lamon-Fava and Fielding 39 , Reference Kien 204 , Reference Kien, Bunn and Ugrasbul 206 , Reference DeLany, Windhauser and Champagne 210 , Reference Jones and Schoeller 211 ). Moreover, in vitro studies have found EPA increases fatty acid oxidation in myotubules( Reference Kalupahana, Claycombe and Moustaid-Moussa 212 , Reference Wensaas, Rustan and Just 213 ).
As the proportion of dietary fatty acids varies within populations and is associated with differences in the fatty acid composition of myocellular membranes, the different types of dietary fatty acids could affect lipid messengers and cellular signalling within the membrane. They could also affect membrane fluidity and the positioning of proteins, as well as levels of muscle ceramide, diacylglycerol, TAG and acylcarnitines( Reference Corcoran, Lamon-Fava and Fielding 39 , Reference Blachnio-Zabielska, Baranowski and Zabielski 214 , Reference Kien, Everingham and Stevens 215 ).
As inflammation is associated with loss of muscle mass in the elderly and since dietary fat composition can influence inflammation, through stimulation of eicosanoid production, dietary fat may influence muscle loss (saturated and trans-fatty acids are considered pro-inflammatory and the n-3 and n-6 fatty acids, anti-inflammatory)( Reference Schaap, Pluijm and Deeg 105 , Reference Kalupahana, Claycombe and Moustaid-Moussa 212 , Reference Kalogeropoulos, Panagiotakos and Pitsavos 216 – Reference Rallidis, Paschos and Liakos 228 ). Fatty acids are associated with insulin resistance in the same direction as inflammation( Reference Honors and Kinzig 6 , Reference Waterlow 229 ).
Total dietary fat (as opposed to individual fatty acids) may also have an effect on muscle mass through a number of mechanisms, including decreased hepatic and skeletal muscle oxidative capacity and by increasing the availability of fatty acids through skeletal muscle for oxidation( Reference Hancock, Han and Chen 230 – Reference Newsom, Schenk and Li 234 ). In animal models, a high-fat diet leads to impaired protein turnover and muscle hypertrophy, through attenuated activation of Akt and S6K1 (kinases in the mTOR pathway)( Reference Sitnick, Bodine and Rutledge 235 ).
Recent human studies have found that supplementation with long chain n-3 PUFA increases rates of protein synthesis and augments the muscle protein anabolic response, and may also attenuate acute muscle loss (cachexia)( Reference Kumar, Kazi and Smith 236 – Reference Fiaccavento, Carotenuto and Vecchini 241 ). One small observational study found a negative association with appendicular lean mass and saturated fat, and a comprehensive study of dietary fat composition and muscle mass found positive associations with the PUFA:SFA ratio and negative associations with saturated and trans fatty acids( Reference Scott, Blizzard and Fell 15 , Reference Welch, MacGregor and Minnihane 81 ).
Antioxidant micronutrients
Oxidative stress and the accumulation of ROS potentially contribute to age-related muscle loss so, consumption of antioxidant nutrients may reduce oxidation in muscle( Reference Liochev 115 ). However, the use of dietary antioxidants for the purpose of attenuating oxidative damage in muscle may not be entirely beneficial as although antioxidants may reduce oxidative damage in mitochondria they also reduce the redox signalling, initiated by ROS, required for muscle contraction( Reference Jackson 119 ).
Of the nutrients considered to be antioxidant (vitamins C, E and carotenoids and the trace elements: Cu, Mn, Se and Zn) the few intervention and observational studies available have mainly investigated the relationship with vitamins C and E. Skeletal muscle is the major body store of vitamin C, estimated to contain up to 67 % of total body vitamin C. Therefore, vitamin C is likely to be essential for muscle structure and function due to its role as an antioxidant and enzyme cofactor for collagen and carnitine synthesis( Reference Carr, Bozonet and Pullar 242 ). A recent intervention study in men found skeletal muscle was highly responsive to increased vitamin C intake with relative uptake being greater in muscle than for leucocytes( Reference Carr, Bozonet and Pullar 242 ).
A number of studies have related decline in physical function or frailty to serum micronutrient concentrations of vitamin E or carotenoids or Se, and also low intake of fruit and vegetables has been associated with functional limitations in mid-life and older men and women( Reference Bartali, Frongillo and Guralnik 243 – Reference Lauretani, Semba and Bandinelli 248 ). One study in women aged 18–79 years found a positive relationship between intake of vitamin C, and total carotene and FFM index and no association with vitamin E and Se( Reference Kelaiditi, jennings and Macgregor 249 ). A further cross-sectional study found that in older women a higher antioxidant nutrient intake (vitamin C, β-carotene, Se) was associated with certain aspects of physical performance (walking and chair rise times)( Reference Martin, Aihie Sayer and Jameson 250 ). One longitudinal observational study found a protective relationship between vitamin C intake and loss of muscle mass in older men and women over 2·6 years( Reference Scott, Blizzard and Fell 15 ). Supplementation studies in human subjects have varied in methodology and in mixtures of nutrients, with one study finding that daily supplementation with 600 mg vitamin E and 1000 mg vitamin C improved FFM when combined with resistance training, more than resistance training alone( Reference Bobeuf, Labonte and Dionne 251 , Reference Labonte, Dionne and Bouchard 252 ). Although there was a small increase in FFM in the group receiving nutritional supplements, only, this was NS( Reference Bobeuf, Labonte and Dionne 251 , Reference Labonte, Dionne and Bouchard 252 ). In rats, supplementation with rutin, vitamins A and E, Zn and Se improved the anabolic response to the amino acid leucine, and reduced inflammation, but the beneficial effects of antioxidants on the leucine response may result from a systemic rather than a localised reduction in oxidative stress and inflammation( Reference Mosoni, Balage and Vazeille 100 , Reference Marzani, Balage and Venien 253 ).
The limited research to date suggests that antioxidant nutrients would be worthwhile investigating further in relation to prevention of muscle loss.
Minerals
Magnesium status has effects on muscle performance, through its roles in energy metabolism and trans-membrane transport and 27 % of magnesium in the body is stored in skeletal muscle( Reference Lukaski 254 , Reference Swaminathan 255 ). Low serum magnesium has been associated with lower muscle strength, but the role of magnesium has so far only been found to be positively related to muscle mass in two studies( Reference Scott, Blizzard and Fell 15 , Reference Kelaiditi, jennings and Macgregor 249 , Reference Dominguez, Barbagallo and Lauretani 256 ). In a 2·6-year follow-up study, magnesium was a positive predictor of change in appendicular lean mass, as was iron, phosphorus and zinc, indicating a role for minerals in conservation of lean mass, i.e. better mineral intake was associated with less muscle loss over time( Reference Scott, Blizzard and Fell 15 ). However, further work is needed in this area.
Bioactive compounds
There is recent research indicating that certain bioactive compounds may influence age-related skeletal muscle loss; however, these have so far only investigated relationships with muscle strength or exercise performance. An olive oil derived anti-oxidant mixture was effective, in aged rodents, in restoring a number of sarcolemma ion channels in muscle( Reference Pierno, Tricarico and Liantonio 257 ). Circumin (a phenol compound found in the spice turmeric) has been used as a supplement in, in vivo studies, in relation to contractile function, although the results have so far been equivocal( Reference Chopard, Hillock and Jasmin 10 ). And finally, a recent review of nitrate supplementation from beetroot juice or inorganic nitrate found results suggestive of a moderate improvement in constant load time to exhaustion tests( Reference Hoon, Johnson and Chapman 258 ). However, the relationship with muscle mass does not yet appear to have been investigated. Bioactive compounds that influence muscle performance and strength may also be relevant for prevention of age-related muscle loss and so deserve further investigation.
Summary and conclusion
The majority of research relating nutrients, other than protein and vitamin D, and muscle loss has been in cross-sectional population studies to date, but the acknowledged issues of potential incomplete adjustment for confounding can exist (differences between people in the factors affecting age-related muscle loss that can be controlled for with statistical analyses). Also, the majority of studies have been in older populations, not the younger or pre-elderly age groups where prevention would be most relevant (given that adequate muscle mass is protective for frailty and mortality and survival from acute bouts of illness that involve muscle loss). Therefore, future research in this area should use randomised controlled trial study designs in younger as well as older populations.
From the available evidence, adequate protein intake is undoubtedly relevant for maintenance of muscle mass during ageing, but the role of other nutrients and other dietary factors such as fat composition, anti-oxidant nutrients, dietary acid–base load, mineral intake and bioactive compounds deserve further research.
Population advice
Current dietary recommendations for prevention of sarcopenia suggest protein intake should be 0·8 g/kg/d (US dietary guidelines Dietary Recommended Allowance) and the maximum intake in the UK is currently set at twice the current reference nutrient intake, i.e. 1·5 g/kg/d which equates to not more than 90 g/d in a woman weighing 60 kg. Recent suggestions are that older adults should consume 1·0–1·2 g/kg/d, although this is debated( Reference Wolfe, Miller and Miller 259 – Reference Waters, Baumgartner and Garry 263 ). It has also been suggested that older people potentially divide protein intake during the day to consume 25–30 g high quality protein per meal in order to maximise protein synthesis( Reference Symons, Sheffield-Moore and Wolfe 264 , Reference Bernstein and Munoz 265 ). At present, there is no recommendation for younger adults although the recommendation to consume 0·8 g/kg/d protein would appear to be wise. Although there is limited research on the relationship between antioxidant nutrients and dietary acid–base load and conservation of muscle mass, consumption of adequate amounts of fruit and vegetables, i.e. following the dietary guidelines for five portions of fruits and vegetables per day would likely contribute to conservation of muscle. Given the potential adverse effects of high alcohol consumption, it would also be advisable to follow UK government guidelines for alcohol consumption (2 units/d for women and 3/d for men). Consumption of foods containing vitamin D and regular, appropriate, exposure to sunlight would also be advisable( Reference Myint and Welch 266 ).
For management of sarcopenia, advice is for total protein intake to be 1·0–1·5 g/kg body weight/d with measurement of 25(OH)D levels and supplementation, where required, to achieve circulating levels of 100 nmol/l( Reference Morley, Argiles and Evans 14 ).
However, the role of diet in the maintenance and prevention of muscle loss in ageing populations needs to be further characterised.
Acknowledgements
The author would like to thank Dr Lee Hooper for critical reading of this paper.
Financial Support
None.
Conflicts of Interest
None.
Authorship
The author was solely responsible for all aspects of preparation of this paper.