Introduction
How individuals change the rise, maintenance, and decline of their affective experiences – the process of emotion regulation – has been widely recognized as a key process from normative functioning to psychopathology (Fernandez, Jazaieri, & Gross, Reference Fernandez, Jazaieri and Gross2016; Gross & Muñoz, Reference Gross and Muñoz1995; Sheppes, Suri, & Gross, Reference Sheppes, Suri and Gross2015). Studies have found that emotion regulation measures can lead to successful predictions of prospective mental health outcomes such as social functioning, well-being, internalizing symptoms, and externalizing symptoms (Berking, Wirtz, Svaldi, & Hofmann, Reference Berking, Wirtz, Svaldi and Hofmann2014; Cameron & Overall, Reference Cameron and Overall2018; Kim & Cicchetti, Reference Kim and Cicchetti2010; Wirtz, Hofmann, Riper, & Berking, Reference Wirtz, Hofmann, Riper and Berking2014). Moreover, recent studies highlight the role of emotion regulation in both the development and successful treatment of diverse dimensions of psychopathology (Aldao, Gee, De Los Reyes, & Seager, Reference Aldao, Gee, De Los Reyes and Seager2016; Fernandez et al., Reference Fernandez, Jazaieri and Gross2016; Sakiris & Berle, Reference Sakiris and Berle2019; Sloan et al., Reference Sloan, Hall, Moulding, Bryce, Mildred and Staiger2017; Weissman et al., Reference Weissman, Bitran, Miller, Schaefer, Sheridan and McLaughlin2019).
Given its relevance in both normative and aberrant affective experiences, increasing number of neuroimaging studies sought to delineate the neural substrates of emotion regulation. In detail, decades of task-based experiments have culminated in relatively reliable meta-analytic mappings of emotion regulation circuits that encompass the amygdala, ventromedial prefrontal cortex, ventrolateral prefrontal cortex, dorsolateral prefrontal cortex, the insula, supplementary motor area, and the cingulate cortex (Buhle et al., Reference Buhle, Silvers, Wager, Lopez, Onyemekwu, Kober and Ochsner2014; Frank et al., Reference Frank, Dewitt, Hudgens-Haney, Schaeffer, Ball, Schwarz and Sabatinelli2014; Kohn et al., Reference Kohn, Eickhoff, Scheller, Laird, Fox and Habel2014; Morawetz, Bode, Derntl, & Heekeren, Reference Morawetz, Bode, Derntl and Heekeren2017). These empirical mappings are also in line with theoretical models that explain emotion regulation as a dynamic process that recruit multiple combinations of cortical–subcortical interactions to shape affective experiences (Braunstein, Gross, & Ochsner, Reference Braunstein, Gross and Ochsner2017; Caballero, Nook, & Gee, Reference Caballero, Nook and Gee2022; Etkin, Büchel, & Gross, Reference Etkin, Büchel and Gross2015; Ochsner, Silvers, & Buhle, Reference Ochsner, Silvers and Buhle2012; Silvers & Moreira, Reference Silvers and Moreira2019; Smith & Lane, Reference Smith and Lane2015).
However, there are two important shortcomings in the relevant literature that need to be addressed: reliance on (1) laboratory-based tasks of emotion regulation and (2) a priori regions of interest (ROIs) approaches. The first limitation of the emotion regulation literature is its heavy reliance on laboratory-based emotion regulation tasks to elucidate the neural substrates supporting successful implementation of certain emotion regulation strategies (Caballero et al., Reference Caballero, Nook and Gee2022; Silvers & Moreira, Reference Silvers and Moreira2019). In other words, there is a relative paucity of neuroimaging research on emotion regulation tendency. Emotion regulation tendency refers to the habitual mode of emotion regulation in which individuals engage in when faced with naturalistic regulatory needs (Silvers & Moreira, Reference Silvers and Moreira2019), and such tendencies cannot be fully captured in laboratory settings where subjects are given certain emotion regulation strategies to utilize. Numerous studies have demonstrated that dispositional patterns through which individuals deploy specific emotion regulation strategies can be especially informative in parsing psychopathology symptoms (Aldao, Nolen-Hoeksema, & Schweizer, Reference Aldao, Nolen-Hoeksema and Schweizer2010; Eftekhari, Zoellner, & Vigil, Reference Eftekhari, Zoellner and Vigil2009; Naragon-Gainey, McMahon, & Chacko, Reference Naragon-Gainey, McMahon and Chacko2017; Sheppes, Scheibe, Suri, & Gross, Reference Sheppes, Scheibe, Suri and Gross2011), but few studies have investigated the neural underpinnings of such patterns. Though a number of studies investigated the neural mechanism behind emotion regulation tendency by giving individuals regulatory choices in experimental settings (Doré, Weber, & Ochsner, Reference Doré, Weber and Ochsner2017; Fine, Schwartz, Hendler, Gonen, & Sheppes, Reference Fine, Schwartz, Hendler, Gonen and Sheppes2022; Shafir, Schwartz, Blechert, & Sheppes, Reference Shafir, Schwartz, Blechert and Sheppes2015; Shafir, Thiruchselvam, Suri, Gross, & Sheppes, Reference Shafir, Thiruchselvam, Suri, Gross and Sheppes2016) or by probing the correlations between self-reported emotion regulation tendency and individual difference in brain activity magnitude (Che, Luo, Tong, Fitzgibbon, & Yang, Reference Che, Luo, Tong, Fitzgibbon and Yang2015; Drabant, McRae, Manuck, Hariri, & Gross, Reference Drabant, McRae, Manuck, Hariri and Gross2009; Kanske, Heissler, Schönfelder, & Wessa, Reference Kanske, Heissler, Schönfelder and Wessa2012; Kanske, Schönfelder, Forneck, & Wessa, Reference Kanske, Schönfelder, Forneck and Wessa2015; Scult, Knodt, Swartz, Brigidi, & Hariri, Reference Scult, Knodt, Swartz, Brigidi and Hariri2017), studying diverse patterns across multiple emotion regulation strategies has been limited because these studies mainly focused on only one or two strategies in experimental settings.
The second limitation of the existing body of research is that the analyses were mostly limited to activations or functional connectivity patterns among a priori ROIs. Extracting neural phenotypes from emotion regulation circuits have led to promising models that predict symptoms or treatment responses (Fournier et al., Reference Fournier, Bertocci, Ladouceur, Bonar, Monk, Abdul-Waalee and Phillips2021; Fresco et al., Reference Fresco, Roy, Adelsberg, Seeley, García-Lesy, Liston and Mennin2017; Klumpp et al., Reference Klumpp, Fitzgerald, Kinney, Kennedy, Shankman, Langenecker and Phan2017; Wu et al., Reference Wu, Liu, Zhou, Feng, Wang, Chen and Wang2022). On the other hand, such models may be further improved by considering whole-brain data to understand the neural correlates of emotion regulation in a more comprehensive manner. Indeed, previous literature highlight that exploring outside the theoretically restricted ROIs contributes to building generalizable models of affective experience (Tejavibulya et al., Reference Tejavibulya, Rolison, Gao, Liang, Peterson, Dadashkarimi and Scheinost2022; Yarkoni & Westfall, Reference Yarkoni and Westfall2017). Evidence that directly support this claim can be found where distributed patterns of activation across the entire brain showed better performance in capturing negative emotions than ROI activations (Chang, Gianaros, Manuck, Krishnan, & Wager, Reference Chang, Gianaros, Manuck, Krishnan and Wager2015), or where subjective fear was represented in distributed systems rather than conventionally defined ‘fear centers’ (Zhou et al., Reference Zhou, Zhao, Qi, Geng, Yao, Kendrick and Becker2021). Taken together, a formal investigation of emotion regulation tendency in the context of whole-brain data is warranted.
One useful approach that would be able to address these issues is to investigate stable networks derived from whole-brain functional connections, or functional connectomes. Accumulating evidence suggest that although transitory states induce significant changes in functional connections (Finn & Bandettini, Reference Finn and Bandettini2021; Geerligs, Rubinov, & Henson, Reference Geerligs, Rubinov and Henson2015; Greene, Gao, Scheinost, & Constable, Reference Greene, Gao, Scheinost and Constable2018), their network organizations are mostly stable within individuals across time (Gratton et al., Reference Gratton, Laumann, Nielsen, Greene, Gordon, Gilmore and Petersen2018; Horien, Shen, Scheinost, & Constable, Reference Horien, Shen, Scheinost and Constable2019; Shen et al., Reference Shen, Finn, Scheinost, Rosenberg, Chun, Papademetris and Constable2017), making them suitable for inspecting trait-like individual differences such as sustained attention (Rosenberg et al., Reference Rosenberg, Finn, Scheinost, Papademetris, Shen, Constable and Chun2016), transdiagnostic psychopathology (Elliott, Romer, Knodt, & Hariri, Reference Elliott, Romer, Knodt and Hariri2018), or fluid intelligence (Finn et al., Reference Finn, Shen, Scheinost, Rosenberg, Huang, Chun and Constable2015). One prime example that emphasizes the utility of connectomes is a recent meta-analytic study using connectomics to find a convergence map that is clinically translatable to effective treatment targeting, which was unattainable when using regional activations (Cash, Müller, Fitzgerald, Eickhoff, & Zalesky, Reference Cash, Müller, Fitzgerald, Eickhoff and Zalesky2023).
On the other hand, structurally bound functional networks have been seldom studied despite its promise in highlighting stable trait-like features. Conceptually, if functional connectivity corresponds to the ‘observed amount of traffic’ between two ‘cities’, white matter structural connectivity derived from diffusion magnetic resonance imaging (dMRI), in turn, corresponds to the ‘highways’ that support the traffic (Amico & Goñi, Reference Amico and Goñi2018). Therefore, one can consider the possibility that stronger networks of highways shaping how traffics operate and include this dynamic in brain connectivity models. Of note, though stronger structural connectivity between two regions promotes functional connectivity between said regions (Sarwar, Tian, Yeo, Ramamohanarao, & Zalesky, Reference Sarwar, Tian, Yeo, Ramamohanarao and Zalesky2021; Sporns, Reference Sporns2011; Suárez, Markello, Betzel, & Misic, Reference Suárez, Markello, Betzel and Misic2020), the structural networks may influence spatially non-overlapping functional networks through facilitating or restricting network-level interactions (Amico & Goñi, Reference Amico and Goñi2018; Mišić et al., Reference Mišić, Betzel, De Reus, Van Den Heuvel, Berman, McIntosh and Sporns2016).
To expand the emotion regulation literature through adopting such network-based approaches, we analyzed the functional connectome in tandem with the structural connectome to search for whole-brain functional–structural network correlates of emotion regulation tendency (Fig. 1). First, as a preliminary examination, we employed an intersubject representational similarity analysis (IS-RSA) framework to test whether individuals with similar functional and structural connectomes have similar emotion regulation tendencies across 23 regulatory strategies (Finn et al., Reference Finn, Glerean, Khojandi, Nielson, Molfese, Handwerker and Bandettini2020). The IS-RSA framework serves as a general test of relationship where statistical assumptions between the variables are minimal, and also retains the high resolution of information in the variables, which is especially helpful when analyzing a set of variables that may be comprised of qualitatively distinct subsets (e.g. 23 emotion regulation strategies). Then, we sought to pinpoint the structurally bound functional networks that represent individual differences in emotion regulation tendency. In detail, we applied independent component analysis (ICA) on the functional–structural hybrid connectome to elucidate the functional–structural covariant components that are reliably present across individuals (Amico & Goñi, Reference Amico and Goñi2018), then subjected these hybrid components to a canonical correlation analysis (CCA) to explore their relationship with emotion regulation tendencies (Smith et al., Reference Smith, Nichols, Vidaurre, Winkler, Behrens, Glasser and Miller2015). We hypothesized that multiple structurally bound functional networks would each be related to diversiform domains of emotion regulation tendency. Lastly, we sought to externally validate the results on a transdiagnostic sample of adolescents from the Healthy Brain Network (HBN; Alexander et al., Reference Alexander, Escalera, Ai, Andreotti, Febre, Mangone and Milham2017) to check if such structurally bound functional networks would also be important in the developmental stage where emotion regulation tendencies as well as psychopathology symptoms purportedly start to emerge (Ahmed, Bittencourt-Hewitt, & Sebastian, Reference Ahmed, Bittencourt-Hewitt and Sebastian2015; Casey, Getz, & Galvan, Reference Casey, Getz and Galvan2008; Lee et al., Reference Lee, Heimer, Giedd, Lein, Šestan, Weinberger and Casey2014; Silvers, Reference Silvers2022; Thompson, Reference Thompson1991).

Figure 1. Schematic of the main analytic framework. The canonical correlation analysis involves the functional–structural hybrid connectome on one end and emotion regulation tendency on the other, both of which were each subjected to dimensionality reduction beforehand. Four independent components emerged from the 268 × 268 functional and structural connectomes, and three principal components were extracted from the 23 dimensions of emotion regulation tendency. Then, these sets of components were entered into the canonical correlation analysis framework to find the configuration of variable weights that determines maximal correlation between the two sets of variables.
Methods and materials
Participants
Data for this study were from the Max Planck Institute ‘Leipzig Study for Mind- Body-Emotion Interactions’ (LEMON) dataset (Babayan et al., Reference Babayan, Erbey, Kumral, Reinelt, Reiter, Röbbig and Villringer2019). The resulting sample comprised of 99 young German-speaking adults (n = 59 in age bracket 20–25, n = 40 in age bracket 25–30) among which 28 were female. Further details on the dataset are described in the online Supplementary Information.
Behavior measures
Data on emotion regulation tendency have been obtained from three different questionnaires (Emotion Regulation Questionnaire, ERQ; Cognitive Emotion Regulation Questionnaire, CERQ; Coping Orientations to Problems Experienced, COPE) to capture a broad range of individual difference in habitual emotion regulation. Details on these questionnaires can be found in the online Supplementary Information.
Connectome building
Image acquisition, preprocessing, and quality check as well as procedures on connectome construction are fully described in the online Supplementary Information. In brief, using the 268-node Shen atlas (Shen, Tokoglu, Papademetris, & Constable, Reference Shen, Tokoglu, Papademetris and Constable2013), structural connectomes were derived from number of streamlines of probabilistic tractography on preprocessed dMRI images (Behrens, Berg, Jbabdi, Rushworth, & Woolrich, Reference Behrens, Berg, Jbabdi, Rushworth and Woolrich2007), while functional connectomes were calculated from correlation coefficients between the mean timeseries of functional activations for each of the 268 regions from preprocessed resting-state functional images (Taylor & Saad, Reference Taylor and Saad2013).
Intersubject representational similarity analyses
IS-RSA via Mantel tests (Finn et al., Reference Finn, Glerean, Khojandi, Nielson, Molfese, Handwerker and Bandettini2020; Mantel, Reference Mantel1967) were performed to examine whether the joint consideration of functional and structural connectomes, compared to either connectome separately, offered better explanatory power for individual differences in emotion regulation tendencies. We therefore tested three intersubject similarity correlation models: (1) interindividual similarity of functional connectome and interindividual similarity of emotion regulation tendencies, (2) interindividual similarity of structural connectome and interindividual similarity of emotion regulation tendencies, and (3) interindividual similarity of functional–structural hybrid connectome and interindividual similarity of emotion regulation tendencies. Details are described in the online Supplementary Information.
Canonical correlation analysis
Given that the functional–structural hybrid connectome showed stronger correlation with emotion regulation tendencies than either the functional or structural connectome by itself, the functional–structural hybrid connectome was further inspected to pinpoint the source of the correlation. Although CCA is a powerful multivariate analytic tool for capturing covariance between two sets of variables with high dimensionality (i.e. 268 × 268 brain variables and 23 behavior variables) (McPherson & Pestilli, Reference McPherson and Pestilli2021; Smith et al., Reference Smith, Nichols, Vidaurre, Winkler, Behrens, Glasser and Miller2015; Wang et al., Reference Wang, Smallwood, Mourao-Miranda, Xia, Satterthwaite, Bassett and Bzdok2020; Xia et al., Reference Xia, Ma, Ciric, Gu, Betzel, Kaczkurkin and Satterthwaite2018), concerns regarding its susceptibility to overfitting has been documented (Dinga et al., Reference Dinga, Schmaal, Penninx, van Tol, Veltman, van Velzen and Marquand2019; Mihalik et al., Reference Mihalik, Chapman, Adams, Winter, Ferreira and Shawe-Taylor2022). To circumvent this issue, we sought to reduce the dimensions of variables before they are entered into the CCA framework, which increases the stability and the reliability of CCA (Dinga et al., Reference Dinga, Schmaal, Penninx, van Tol, Veltman, van Velzen and Marquand2019; Mihalik et al., Reference Mihalik, Chapman, Adams, Winter, Ferreira and Shawe-Taylor2022; Wang et al., Reference Wang, Smallwood, Mourao-Miranda, Xia, Satterthwaite, Bassett and Bzdok2020). Crucially, considering the number of subjects of our study (n = 99), the number of variables that would lead to reliable results is around 3–10 according to introductory texts (Pituch & Stevens, Reference Pituch and Stevens2015; Tabachnick & Fidell, Reference Tabachnick and Fidell2001) and less than 9 according to a recent methodological guide (Mihalik et al., Reference Mihalik, Chapman, Adams, Winter, Ferreira and Shawe-Taylor2022). Therefore, these standards were kept in mind when deciding the optimal number of dimensions, which are outlined in the online Supplementary Information. As a result, four independent components of functional–structural hybrid connectomes and three principal components of emotion regulation tendency were identified.
CCA takes two sets of variables and finds the optimal configuration of variable weights that maximizes the correlation between the two sets of variables. The analytic framework and the code for the CCA analysis followed a previous work (Smith et al., Reference Smith, Nichols, Vidaurre, Winkler, Behrens, Glasser and Miller2015), which also utilized CCA to pinpoint brain–behavior links after carrying out dimension reduction schemes on datasets to ensure reliability. First, for each participant, their weight for each of the four ICA components were found by averaging the weight values across the 1000 runs. Second, a covariate matrix was derived by aggregating and normalizing data on gender, age, height, weight, heart rate, resting-state EPI movement, and diffusion-weighted imaging movement for all subjects. Gender and age (in 5-year range brackets) were entered as binary dummy-codes, and data on height (cm) and weight (kg) were provided up to the first decimal figure. Heart rate data were collected during resting-state scans using BIOPAC MP150 acquisition system (BIOPAC Systems Inc., Goleta, CA, USA) and the acquisition software AcqKnowledge (Version 4.0, BIOPAC Systems Inc.), and the systole and the diastole signal at the brachial artery of the left arm were each used as covariates. Mean FD values during the resting-state acquisition and mean relative movement parameters during the diffusion image acquisition were also entered as covariates. Third, the covariate matrix was regressed out from both the functional–structural hybrid ICA components and the emotion regulation tendency PCA components. Fourth, CCA (cancorr function in Matlab) was run 10 000 times with the emotion regulation tendency PCA components randomly permuted across subjects each time. This formed the null distribution of CCA results, on which the non-parametric significance of the true CCA result could be tested. Lastly, the true CCA result was tested for significance with the p < 0.05 threshold after multiple test correction.
Control analyses
To account for possible alternate explanations for our results, we carried out multiple control analyses to test if the results were driven by (1) the functional connectome, (2) the structural connectome, or (3) the pure amount of data in the functional and structural connectome. Details can be found in the online Supplementary Information.
External validation on transdiagnostic adolescent data
Based on prior literature that emphasize the role of adolescence in development of emotion regulation tendencies and psychopathology (Ahmed et al., Reference Ahmed, Bittencourt-Hewitt and Sebastian2015; Casey et al., Reference Casey, Getz and Galvan2008; Lee et al., Reference Lee, Heimer, Giedd, Lein, Šestan, Weinberger and Casey2014; Silvers, Reference Silvers2022; Thompson, Reference Thompson1991), the HBN dataset was used to probe if the results of our CCA analyses were generalizable to adolescent subjects with varying psychiatric diagnoses. As a direct correlate of emotion regulation tendency, we inspected coping strategies from the Children's Coping Strategies Checklist – Revised (CCSC-R1; Ayers et al., Reference Ayers, Sandler, Bernzweig, Harrison, Wampler and Lustig1989). We additionally probed experience of positive affect as a possible consequence of emotion regulation, gauged by the Positive And Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, Reference Watson, Clark and Tellegen1988).
First, adolescent subjects with psychiatric diagnoses were selected from the HBN dataset Releases 7 through 10. Excluding subjects with insufficient data, no diagnosis, or with head movement above 0.2 mm in mean FD, the final sample consisted of 93 adolescents aged 11–19 years (34 females; mean age 14.39 ± 2.12). The sample was fully transdiagnostic as the adolescents were diagnosed with at least one psychiatric illness. According to diagnoses, the adolescents were further categorized into internalizing disorder (n = 38; Generalized Anxiety Disorder, Major Depressive Disorder, Obsessive-Compulsive Disorder, Bulimia Nervosa, Persistent Depressive Disorder, Social Anxiety, and Specific Phobia), externalizing disorder (n = 2; Conduct Disorder-Childhood-onset type and Alcohol Use Disorder), and neurodevelopmental disorder (n = 53; ADHD-Combined Type, ADHD-Inattentive Type, Autism Spectrum Disorder, Borderline Intellectual Functioning, Intellectual Disability, Language Disorder, and Learning Disorder).
Functional connectomes of the adolescents were constructed following procedures reported in the online Supplementary Information. External validation was carried out by calculating network scores to explore the generalizability of the originally found network. We adopted this approach to discover a generalizable network model of emotion regulation tendencies that can aid further research without having to collect extensive emotion regulation tendency data or construct computationally expensive structural connectomes every time. One notable example is the sustained attention network model (Rosenberg et al., Reference Rosenberg, Finn, Scheinost, Papademetris, Shen, Constable and Chun2016), which has been leveraged to uncover novel findings pertaining to the nature of attention and related constructs – all based on a network predefined from a discovery sample (Jangraw et al., Reference Jangraw, Gonzalez-Castillo, Handwerker, Ghane, Rosenberg, Panwar and Bandettini2018; Kardan et al., Reference Kardan, Stier, Cardenas-Iniguez, Schertz, Pruin, Deng and Rosenberg2022; Rosenberg et al., Reference Rosenberg, Scheinost, Greene, Avery, Kwon, Finn and Chun2020). Nonetheless, as it is critical to also establish the replicability of our findings, we also constructed the structural connectomes and employed the same CCA framework in the adolescent dataset as described in the online Supplementary Information. Moreover, although not the primary aim of the validation analyses, we sought to confirm that the structural network found in the young adult dataset was also generalizable. We therefore calculated the structural network score by applying the methods described above to the structural connectomes of the adolescents and tested it for behavioral relevance.
We calculated the composite functional network score by first multiplying the weights of the final composite functional network from the young adult dataset with the functional connectomes of each subject in the adolescent dataset, and then aggregating the absolute values across the entire connectome to find single-value scores for each individual. These network scores would be higher for subjects with stronger positive and negative connections across the composite functional network, and lower for subjects with overall weaker connections across the network. Notably, because individuals with stronger connections but with opposite signs (e.g. values of 3 and −2 for subject A and −3 and 2 for subject B) would be assigned the same absolute sum score (e.g. score of 5 for both subjects), we additionally calculated a positive and negative network score for each individual by aggregating across all positive or negative values only.
Then, Pearson correlations were conducted between these composite functional network scores and positive affects score and coping tendency score. Positive affect score was derived from 10 questions from the PANAS scale where subjects are prompted to answer the extent to which they generally feel a given emotion (e.g. ‘Interested’, ‘Excited’, ‘Strong’) on a 5-point Likert scale (PANAS; Watson et al., Reference Watson, Clark and Tellegen1988). Coping tendency score was defined as the first principal component of the 13 coping strategies from the CCSC scale, which was found sufficient by the same criteria described above (Lüdecke, Ben-Shachar, Patil, & Makowski, Reference Lüdecke, Ben-Shachar, Patil and Makowski2020), explaining 46.0% of variance from the scale. Additionally, we have examined the relevance of our network feature with regards to psychiatric symptoms from the Child Behavior Checklist (CBCL; Achenbach, Reference Achenbach1991).
Because the composite functional network absolute sum score was positively correlated with both behavioral measures, we also carried out a path analysis. In a path analytic framework, there is a direct path and an indirect path between an independent variable and a dependent variable. We reasoned that if the composite functional network can be replicated in the adolescent age, it will be correlated to the general experience of positive affect through promoting use of adaptive strategies. Therefore, a direct path from the composite functional network score to positive affect as well as an indirect path including the coping strategies principal component were tested. After finding evidence of indirect effects through linear modeling, bootstrapping of 5000 trials was conducted to measure the 95% confidence interval of the tests via the Causal Mediation Analysis package in R (Tingley, Yamamoto, Hirose, Keele, & Imai, Reference Tingley, Yamamoto, Hirose, Keele and Imai2014).
Results
Intersubject representational similarity analysis
The functional–structural hybrid connectome showed stronger correlation with emotion regulation tendencies than either the functional or structural connectome by itself, supported by various tests outlined in the online Supplementary Information.
Canonical correlation analysis
With three principal components of emotion regulation tendency and four independent components of functional–structural hybrid connectome as input, CCA resulted in one significant mode of correlation after 10 000 permutations (r = 0.336, p = 0.025 after correcting for multiple tests). The significant mode of correlation was between an adaptive-to-maladaptive gradient of emotion regulation tendency and the functional–structural network which mainly involved the functional and structural connections of the visual cortex. To elaborate, the three principal components of emotion regulation tendency with the weight of −0.125, −0.835, and −0.530 showed a canonical correlation with the four independent components of functional–structural hybrid connectome with the weight of 0.275, 0.359, −0.940, and −0.025. Each of these components, multiplied by their weights, was summed to create a comprehensive brain–behavior correlation that is interpretable.
As a result, the composite emotion regulation tendency mode revealed an adaptive-to-maladaptive gradient with active coping, positive reframing, and positive reappraisal on one end, and self-blame (both COPE and CERQ) and catastrophizing on the other (Table 1). The composite functional–structural hybrid model had pronounced functional connections concentrated in the motor network and the visual networks (Fig. 2a) as well as structural connections most heavily involving the subcortical–cerebellum network and the default mode network (Fig. 2b). In detail, the adaptive-to-maladaptive emotion regulation tendency gradient (1) negatively covaried with functional connections in the default mode network and the subcortical–cerebellum network, (2) positively covaried with functional connections involving the motor network and the visual networks, (3) negatively covaried with structural connections in the subcortical–cerebellum network and the default mode network, (4) positively covaried with structural connections among the motor network and the visual networks.
Table 1. Composite emotion regulation tendency from the significant mode of canonical correlation

The 23 emotion regulation strategies are shown ordered by their canonical correlation weight.

Figure 2. Composite functional and structural network properties from the significant mode of canonical correlation. (a) Functional connections of the significant mode reveal concentration in the motor network and the visual networks. Top left figure denotes important nodes with size and color scaled by eigenvector centrality, a metric of graph centrality emphasizing nodes that are connected to other nodes with high eigenvector centrality that was calculated via Brain Connectivity Toolbox (Rubinov & Sporns, Reference Rubinov and Sporns2010). Important connections that carry top 1% weight in either the positive or the negative direction are also shown. The same important connections are signified on the top right 268 × 268 matrix, ordered by eight network labels (Shen et al., Reference Shen, Tokoglu, Papademetris and Constable2013). Bottom left and right figures dissociate the connections that either positively covaries or negatively covaries with the adaptive-to-maladaptive gradient of emotion regulation tendency. (b) Structural connections of the significant mode illustrate relevance of subcortical–cerebellar structures and default mode network connections.
Control analyses
The results of the control analyses described in the online Supplementary Information ensured that the CCA results were not solely dependent on (1) the functional connectome, (2) the structural connectome, or (3) the pure amount of data in the functional and structural connectome.
External validation on transdiagnostic adolescent data
We focused on the first principal component of the coping strategies that roughly followed an adaptive-to-maladaptive spectrum from ‘Cognitive Decision Making’ and ‘Optimistic Thinking’ to ‘Repression’ and ‘Avoidant Actions’ (Table 2; accounting for 46.0% variance) and named this component the coping tendency score. The composite functional network score was positively correlated with this coping tendency score (r = 0.236, p = 0.023) as well as positive affect from the PANAS scale (r = 0.270, p = 0.009; Fig. 3a). Aside from the network absolute sum score used above, network positive value score and network negative value score were also tested for correlation with positive affect and coping tendency score. The positive network score was positively correlated with coping tendency score (r = 0.294, p = 0.004) and positive affect (r = 0.242, p = 0.019), while the negative network score was not significantly correlated with coping tendency score (p = 0.511) or positive affect (p = 0.153). Despite the positive network score carrying heavier behavioral relevance, the absolute sum score was used for further analyses because it is conceptually more in line with our network property in which positive and negative connections work together. The structural network score was not significantly correlated to positive affect (p = 0.711) or the coping tendency score (p = 0.114). The functional composite network score was not correlated with the internalizing symptom score (p = 0.193) or the externalizing score (p = 0.991), and the same was true for the structural composite network score (p = 0.560; p = 0.561).
Table 2. One principal component of Children's Coping Strategy Checklist
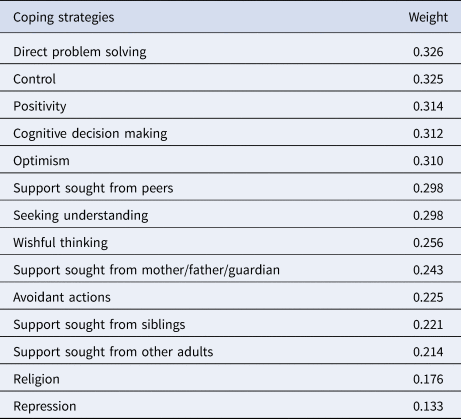
The 14 coping strategies are shown ordered by their principal component weight.

Figure 3. External validation on a transdiagnostic adolescent dataset. (a) Composite functional network score is positively correlated with positive affect and coping tendency. Composite functional network score was calculated by multiplying the weights from the network property of the young adult dataset with the connectomes of the adolescents and then finding the sum of the absolute values across each connectome. Positive affect was assessed via PANAS scale, and coping tendency score was defined from extracting the first principal component of the CCSC Questionnaire. The adolescents were categorized into internalizing, externalizing, or neurodevelopmental disorders according to their diagnoses, indicated as colors of dots on scatterplots. (b) The effect of the composite functional network score on positive affect is fully explained by the coping tendency in transdiagnostic adolescents. 95% Confidence intervals that do not include zero signify statistical significance. *p < 0.05; **p < 0.01; ***p < 0.001.
In the following path analysis, the effect of the composite functional network score on positive affect was fully explained by the coping tendency score (Fig. 3b). In other words, the path from the composite functional network score to coping tendency score (B = 0.0005, p = 0.023) and the path from coping tendency score to positive affect were both significant (B = 1.6218, p < 0.001), but the direct effect on positive affect from the composite functional network score (B = 0.0020, p = 0.009) was not significant anymore when considering the indirect path that included the coping tendency score (B = 0.0012, p = 0.083). Significance testing through 5000 trials of bootstrapping revealed that the indirect path was significant (indirect effects = 0.0008, 95% CI [0.00005–0.0016]), whereas the direct path was not (direct effects = 0.0012, 95% CI [−0.0001 to 0.0026]).
Discussion
In our study, IS-RSA and CCA have each demonstrated that individual differences in emotion regulation tendency can be gleaned from whole-brain functional and structural connections. First, IS-RSA showed that emotion regulation tendency is best captured when investigating both the structural and the functional connectome. Second, CCA results associated an adaptive-to-maladaptive gradient of emotion regulation tendency with a network feature conveying noticeable contributions from motor network and visual network functional connections as well as default mode network and subcortical–cerebellum network structural connections. Together, our study advances the theoretical understanding of emotion regulation tendency by elucidating its neural foundations while also confirming multiple streams of clinical research on the transdiagnostic psychopathology factor.
First, our results provide affirmation as well as novel insights on the theoretical models of affective processes. Connections involving the visual network regions, motor network regions, and cerebellar regions, which are traditionally placed at the lowest end of the regulation hierarchy model (Smith & Lane, Reference Smith and Lane2015), were most strongly correlated with the adaptive-to-maladaptive gradient of emotion regulation tendency. This implies that (1) individual differences in the network organization that oversees the somatosensory stages of emotion generation may cause downstream differences in voluntary emotion regulation tendency, or (2) adaptive-to-maladaptive emotion regulation tendency may be embedded in low-level networks. The former explanation is in line with the hierarchical models of emotion regulation, where the low-level functionalities of the human brain related to basic body states and movements form the building blocks of emotion generation that lead to downstream identification, choice, and implementation of regulatory goals (Gross, Reference Gross and Gross2014; Sheppes et al., Reference Sheppes, Suri and Gross2015; Smith & Lane, Reference Smith and Lane2015). Within such frameworks, our result may indicate that individual differences in low-level processes that are ostensibly devoid of affective value can bring on differences in habitual emotion regulation tendency down the line. The latter, on the other hand, relates to relatively recent findings that affective information such as valence (Bo et al., Reference Bo, Yin, Liu, Hu, Meyyappan, Kim and Ding2021; Kragel et al., Reference Kragel, Čeko, Theriault, Chen, Satpute, Wald and Wager2021; Xu et al., Reference Xu, Zhang, Li, Zhou, Lin, Zhang and Liang2023), fear learning (Li & Keil, Reference Li and Keil2023; You, Brown, & Li, Reference You, Brown and Li2021), distinct emotion categories (Kragel, Reddan, LaBar, & Wager, Reference Kragel, Reddan, LaBar and Wager2019), and even emotion down-regulation (Bo et al., Reference Bo, Kraynak, Kwon, Sun, Gianaros and Wager2023) is processed at the rudimentary visual perception stage. Building on such empirical evidence, we may speculate that early stages of affective experience may already contain information on regulatory tendency that spans an adaptive-to-maladaptive gradient. Though both accounts are plausible, our study demonstrates a clear link between low-level network features and high-level emotion regulation tendencies, speaking to the importance of conceptualizing the emotion regulation process as an integrated and dynamic system instead of a segregated and unidirectional system that follows a strict hierarchy (Pessoa, Reference Pessoa2017; Underwood, Tolmeijer, Wibroe, Peters, & Mason, Reference Underwood, Tolmeijer, Wibroe, Peters and Mason2021).
Second, our network property also aligns well with a burgeoning literature on neural correlates of the transdiagnostic factor of psychopathology. On one hand, adaptive or maladaptive emotion regulation tendency has been consistently implicated with multiple psychopathology symptoms across diagnoses (Aldao et al., Reference Aldao, Nolen-Hoeksema and Schweizer2010; Eftekhari et al., Reference Eftekhari, Zoellner and Vigil2009; Fernandez et al., Reference Fernandez, Jazaieri and Gross2016; Naragon-Gainey et al., Reference Naragon-Gainey, McMahon and Chacko2017). On the other, studies aiming to locate the neural basis of general psychopathology have also been accumulating evidence supporting the involvement of regions and networks prominently featured in the present data. In detail, while abnormal connectome-wide functional connectivity of the visual association cortex and default mode network have been found to be indicative of transdiagnostic psychopathology features (Doucet et al., Reference Doucet, Janiri, Howard, O'Brien, Andrews-Hanna and Frangou2020; Elliott et al., Reference Elliott, Romer, Knodt and Hariri2018; Whitfield-Gabrieli & Ford, Reference Whitfield-Gabrieli and Ford2012), functional connectivity disruptions of somatosensory–motor network both within and across networks have been documented as transdiagnostic signatures of psychopathology (Kebets et al., Reference Kebets, Holmes, Orban, Tang, Li, Sun and Yeo2019). Moreover, general psychopathology features related to structural connectivity and gray matter volume alterations of cerebellar circuitry and visual cortices have been demonstrated and replicated with large transdiagnostic datasets (Moberget et al., Reference Moberget, Alnæs, Kaufmann, Doan, Córdova-Palomera, Norbom and Westlye2019; Romer et al., Reference Romer, Knodt, Houts, Brigidi, Moffitt, Caspi and Hariri2018, Reference Romer, Knodt, Sison, Ireland, Houts, Ramrakha and Hariri2021b). Although these studies did not explicitly connote emotion regulation tendency as a transdiagnostic risk factor, maladaptive regulatory goals have been pointed out as the primary consequence of such transdiagnostic neural aberrations (Romer, Hariri, & Strauman, Reference Romer, Hariri and Strauman2021a). To sum up, our network property that is correlated with an adaptive-to-maladaptive gradient of emotion regulation tendency resembles previous findings that highlight brain-wide functional and structural connectivity anomalies as signatures of transdiagnostic psychopathology.
Finally, our composite network related to emotion regulation tendency was externally validated in a transdiagnostic sample of adolescents aged 11–19. In detail, adolescents with stronger signatures of the composite functional network from the young adult sample had higher general experiences of positive affect through more frequent use of adaptive coping strategies such as ‘Direct Problem Solving’ or ‘Positivity’. As emotion regulation tendencies that start to be fully fleshed out in adolescence can act as risk or resilience toward psychopathology, locating a neural marker of emotion regulation tendency has implications for preventive and early intervention (Cracco, Goossens, & Braet, Reference Cracco, Goossens and Braet2017; Silvers, Reference Silvers2022; Young, Sandman, & Craske, Reference Young, Sandman and Craske2019). It is also noteworthy that such effects were found in a sample of adolescents with distinct psychiatric diagnoses that span internalizing, externalizing, and neurodevelopmental disorders. One potentially fruitful avenue of translational research is to target our network related to emotion regulation tendency as a neural marker of resilience in pediatric psychiatric patients that may be associated to positive affect through use of more adaptive coping strategies, which in turn could mitigate symptoms (Davis & Suveg, Reference Davis and Suveg2014; Gilbert, Reference Gilbert2012).
Together, our results imply that the whole-brain network that was extracted from young adults that was linked to an adaptive-to-maladaptive spectrum of emotion regulation tendencies starts to take shape as early as adolescence. That said, carrying out the identical CCA analyses on the adolescent dataset produced a network that had different configurations of important connections though the connections similarly involved the visual networks and the subcortical–cerebellar network. At least three possible explanations exist for this. First, the specific configurations of connections critical to the adaptive-to-maladaptive emotion regulation tendency may manifest differently for individuals at distinct developmental stages or mental health states. Second, the sub-critical connections in the network may cumulatively explain the adaptive-to-maladaptive emotion regulation tendency, given the fact that the young adult network was generalized to the adolescents despite differences in their most important connections. Third, the CCSC scale may not have captured the full extent of the adaptive-to-maladaptive emotion regulation tendency spectrum that was originally acquired from three different questionnaires. While these possibilities remain open, future work could leverage the generalizable network found in our study to explore such outstanding questions.
There are still limitations to our study. First, our network feature that represents an adaptive-to-maladaptive emotion regulation tendency was defined at a whole-brain scale, but multiple subsystems could theoretically be present within this network feature. This prospect adheres to a phenomenon well-documented regarding the default mode network where multiple functionally segregated subsystems were found to be embedded within the network (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, Reference Andrews-Hanna, Reidler, Sepulcre, Poulin and Buckner2010; Buckner & DiNicola, Reference Buckner and DiNicola2019). Such a possibility also aligns with our ICA results where robust independent components reached a plateau at four and then at 16, which could imply that the four components were recapitulated as fragmented representations. Although our study was not designed to pinpoint individual differences at such minute scale, whether the fine-grained subsystems within our network related to emotion regulation tendency could aid in parsing heterogeneity in emotion regulation and psychopathology is a question that remain unexplored. Second, our measure of emotion regulation tendency depends on cross-sectional self-report questionnaires, which may not thoroughly cover the dynamic nature of emotion regulation tendency that persist throughout daily life (Lincoln, Schulze, & Renneberg, Reference Lincoln, Schulze and Renneberg2022). That said, we used a conglomerate of three different emotion regulation strategy questionnaires to extract coherent patterns from expansive repertoires of strategies. Future studies could use ecological momentary assessment to track multiple features of emotion regulation such as frequency or flexibility in relation to the neural foundations of emotion regulation (Colombo et al., Reference Colombo, Fernández-Álvarez, Suso-Ribera, Cipresso, Valev, Leufkens and Botella2020). Third, females were underrepresented in our main sample of young adults (<30%). Despite including gender as a covariate in the main analytic framework, our analyses were likely ill-equipped to determine potential effects of gender, which have been reported to exist in diverse facets of emotion regulation (Nolen-Hoeksema, Reference Nolen-Hoeksema2012). We therefore remain cautious in terms of making claims about the generalizability of our results across genders. Collecting ample sample size across genders would be a crucial goal for future studies, as gender-related differences in neural activity have also been documented in the traditional cortical–subcortical pathways (Mak, Hu, Zhang, Xiao, & Lee, Reference Mak, Hu, Zhang, Xiao and Lee2009). Fourth, the relatively low loadings on the emotion regulation tendency principal components in the young adult sample as well as the coping strategy principal component in the adolescent sample suggests that the strategies are not as highly correlated as expected. While employing as many variables as possible and preserving the multivariate nature of the emotion regulation strategies could lead to more exhaustive explanations of the possible brain–behavior relationships, we chose to adhere to parsimonious models that would prevent overfitting while maximizing generalizability. That said, we note that replacing the single coping tendency component with the first three principal components increased the effect in the generalization analyses (i.e. better model fit), accounting for the full variability of the emotion regulation tendencies and the coping strategies without any dimensionality reduction may yield stronger and clearer effects. Though comparing the performance between similar multivariate methods falls outside the aim of the current study, more refined dimensionality reduction or regularization methods that retain the most amount of meaningful variability such as domain-driven dimension reduction may grant supplementary insights (Liu, Whitaker, Smith, & Nichols, Reference Liu, Whitaker, Smith and Nichols2022; for comparison among methods, see also Mihalik et al., Reference Mihalik, Chapman, Adams, Winter, Ferreira and Shawe-Taylor2022). Lastly, only the functional composite network score was generalized to the transdiagnostic adolescent sample, and not the structural composite network score. This may indicate that the functional and structural connectomes in the transdiagnostic adolescent sample are not coupled in an identical manner as the young adults, and also that only the functional network is generalizable to the coping strategies and positive affect of the adolescents. It is noteworthy that previous literature suggest that adolescence may be a time that structural–functional coupling goes through cortex-wide changes to support functional development (Baum et al., Reference Baum, Cui, Roalf, Ciric, Betzel, Larsen and Satterthwaite2020; Park et al., Reference Park, Paquola, Bethlehem, Benkarim, Mišić and Bernhardt2022). However, formal tests using a healthy adolescent sample and a young adult sample with psychiatric symptoms may need to take place to fully flesh out such interpretations.
Conclusion
Our study demonstrates the existence of a network related to an adaptive-to-maladaptive gradient of emotion regulation tendency that span the entire brain of functional and structural connections. Elucidating the topology of this network system can inform existing theories on affective experiences while also expanding our understanding of the neural mechanisms underlying pediatric psychopathology. Together, our findings complement theory-driven neural schematics of emotion regulation with a network feature identified through a data-driven approach.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291724000473
Data availability
The MPI-LEMON dataset (https://www.nitrc.org/projects/mpilmbb) (https://doi.org/10.18112/openneuro.ds000221.v1.0.0) is publicly available. The HBN dataset is also publicly available (http://fcon_1000.projects.nitrc.org/indi/cmi_healthy_brain_network/). Analytical pipeline and scripts have been deposited in GitHub (https://doi.org/10.5281/zenodo.7885176).
Acknowledgements
We thank the original authors of the MPI-LEMON dataset and the HBN dataset for their generosity in making it available for use.
Funding statement
This research was supported by the National Research Foundation of Korea (NRF-2021R1F1A1045988). The authors report no potential conflicts of interest.








