Kashin-Beck disease (KBD) is a chronic endemic osteoarthropathy with articular cartilage degeneration and necrosis. It occurs predominately in the northern part of China, Russia and a few northern areas of North Korea(Reference Yang1). Presently, the aetiology is not clear. Epidemiological study has revealed that low Se may play a crucial role in the pathogenesis of KBD(Reference Wang2–Reference Wang, Guo and Duan4). Essential trace element Se exerts its biological functions in vivo mainly through selenoproteins. Selenoprotein P (SEPP1) is one of the rather special selenoproteins. In recent years, the study of SEPP1 is receiving increasing attention, especially the possible association between genetic polymorphisms of SEPP1 and disease susceptibility.
Recently, two relatively common single nucleotide polymorphisms (SNP) of SEPP1 have been reported. It was shown that one SNP, which is located in the 3′ untranslated region (position 25 191) may predict the behaviour of biomarkers of Se status and response to supplementation and thus susceptibility to the disease(Reference Meplan, Crosley and Nicol5). The results obtained from the studies done on trypanosomiasis indicate that SEPP1 plays a role in protection against infectious illnesses(Reference Bosschaerts, Guilliams and Noel6). Three variants of SEPP1 gene (rs3797310, rs2972994, − 4166) were significantly associated with advanced adenoma risk(Reference Rayman7).
Selenocysteine is the active form of Se that is responsible for various important biological functions of SEPP1. Furthermore, the SEPP1 mRNA contains two functional selenocysteine elements in its 3′ untranslated region(Reference Berry, Banu and Harney8). Normally, the SEPP1 is down-regulated in a subset of human prostate tumours, mouse tumours and prostate carcinoma cell lines(Reference Alfonso, Nianqing and Jason9). There is a significant reduction or loss of SEPP1 mRNA expression in colon cancers(Reference Volker10). Although all these genetic studies suggest a possible association of some SEPP1 SNP in individuals with some disease, the association between SEPP1 gene polymorphism and susceptibility to KBD and the SEPP1 mRNA expression level in different tissues have not been studied.
Therefore, the present study was designed to investigate the role of polymorphism in the 3′ untranslated region of the SEPP1 gene, which is at position 25 191 and described as r25191g/a, in KBD patients in endemic areas of Shaanxi province of China, and to detect the SEPP1 mRNA level in whole blood and articular cartilage tissue of the KBD patients and healthy subjects, respectively.
Materials and methods
Study population
The study group consisted of 167 KBD patients (eighty-nine males and seventy-eight females; mean age 52·1 (sd 5·4) years) and 166 healthy controls (eighty-one males and eighty-five females; mean age 52·2 (sd 4·6) years), who were all Han Chinese and were from the same geographical area (Shannxi, China). KBD was diagnosed on the basis of clinical and radiological findings according to the national diagnostic criteria of KBD. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Human Ethics Committee of Xi'an Jiaotong University, People's Republic of China. Written informed consent was obtained from all the subjects.
The blood samples were collected from the KBD and control groups. Articular cartilage tissue was also obtained from knee joints from a total of nine KBD patients (during excision of corpus liberum) and three control subjects (during joint replacement surgery).
Genotype analysis
Genomic DNA was isolated from whole-blood samples from 167 KBD patients and 166 unrelated volunteers, and was extracted by the traditional phenol/chloroform extraction method. Optimisation of tetra-primer amplification refractory mutation system PCR (ARMS PCR) for the detection of SNP r25191g/a was done empirically using the primers designed by the original software available on the website: http://cedar.genetics.soton.ac.uk/public_html/primer.html. The PCR primer sequences were as follows: forward inner primer (G allele), 5′-TGACCTTCAAACTAAATATTTAAAATCGG-3′, and reverse inner primer (A allele), 5′-TGTGTCTAGACTAAATTGGGGAGTATTTT-3′; forward outer primer (5′–3′), GAGGAGAACATAACTGAATCTTGTCAGT-3′, and reverse outer primer (5′–3′), 5′-CTCCATCATAAAAAATATGGTTTGAGTC-3′. The G and A alleles at SNP r25191g/a in the 3′ untranslated region of the SEPP1 gene were identified using the tetra-primer ARMS PCR methodology. Two primers recognised ‘G’ and ‘A’ alleles, and the other two primers were used for inner control. PCR was carried out using an Eppendorf gradient type mastercycler (Eppendorf, Germany) with a total volume of 12·5 μl, containing 6·25 μl of 2 × Master Mix, 0·5 μl of each primer (10 μm), 1·5 μl of genomic DNA and 2·75 μl of H2O. PCR were performed under the following conditions: 95°C for 3 min, followed by thirty-five cycles at 94°C for 30 s, annealing at 57°C for 30 s and extension at 72°C for 30 s, and final elongation step at 72°C for 5 min.
The amplified ARMS PCR products (PCR product size: GG = 213 bp and AA = 183 bp, and PCR control fragment size: 338 bp) were identified by gel electrophoresis, which was carried out on 2 % agarose gels stained with ethidium bromide (0·5 μg/ml).
Quantitative real-time PCR
Real-time PCR was used to assess SEPP1 mRNA expression from a total of twenty whole-blood samples randomly (KBD: 10, and control: 10) and twelve articular cartilage tissue samples (KBD: 9, and control: 3).
Total RNA was isolated from 3 ml of whole blood and 100 mg of articular cartilage tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Total RNA were reverse transcribed using the RevertAid™ First-Strand cDNA Synthesis Kit (MBI, Fermentas, Vilnius, Lithuania) according to the manufacturer's instructions to obtain first-strand complementary DNA.
The mRNA quantification of SEPP1 in whole blood and articular cartilage tissue was performed on iQ™5 Real-Time PCR Detection Systems (Bio-Rad, Philadelphia, PA, USA) using BioEasy SYBR Green I Real-Time PCR Kit (Bioer, Hangzhou, People's Republic of China) according to the manufacturer's instructions. Amplification primers that were used are as follows: SEPP1 (forward: 5′-GATGGAGCAACTGAAAGGTG-3′, and reverse: 5′-CCCCTAGGTCATAGTTTACG-3′)(Reference Alfonso, Nianqing and Jason9) and β-actin, internal standard (forward: 5′-GAACGGTGAAGGTGACAGCAG-3′, and reverse: 5′-GTGGACTTGGGAGAGG ACTGG-3′). Reactions were performed in a 20 μl mixture containing 2 μl of complementary DNA, 0·5 μl of each primer (10 μm), 12·5 μl of 2 × SYBR Mix (with 4·0 mm Mg2+), 0·15 μl of Taq DNA Polymerase and 9·35 μl of double distilled H2O under the conditions of initial denaturation at 94°C for 2 min, followed by forty cycles of denaturation at 94°C for 10 s, annealing at 58°C for 15 s and extension at 72°C for 30 s. All reactions were performed in duplicate. Results were normalised with total levels of β-actin expression, and analysed using iQ™5 software (version 2.0; Bio-Rad) and SPSS 13.0 (SPSS, Chicago, IL, USA).
Statistical analysis
All data were analysed using SPSS software version 13.0. Deviation from the Hardy–Weinberg equilibrium was analysed by χ2 goodness-of-fit test. The distribution normality was analysed using the Kolmogorov–Smirnov test. Differences without skewness between the KBD and control groups were assessed by two-tailed Student's t test. Categorical variables were presented using frequency counts and compared by χ2 test. All P-values were of two ways at 0·05 % level of significance. The risk of developing the disease was expressed as OR and 95 % CI.
Results
Genotype analysis
To evaluate the accuracy of the assigned SEPP1 genotypes, a total of fifty samples were randomly chosen, and the results were confirmed by repeating the ARMS PCR. Notably, no discrepant results were detected when the ARMS PCR assay was repeated using the same samples. The genotype distribution of the SNP r25191g/a in patients with KBD and the control group was in the Hardy–Weinberg equilibrium (P>0·05).
In the present study, we analysed SNP r25191g/a in 333 individuals. The genotype and allele frequencies of individuals for SNP r25191g/a were determined. As shown in Table 1, no significant difference in the distribution of the genotype of SNP r25191g/a was found between the individuals with KBD and controls (50·9 % G/G, 40·1 % G/A and 9·0 % A/A v. 59·0, 32·0 and 9·0 %, respectively, P = 0·29), but the genotype frequency of GA heterozygote tended to be higher in patients with KBD than in the controls. The distribution of the A allele frequencies was also not different between cases and controls (29·0 v. 25·0 %, respectively, P = 0·428). The OR value showed no association between the SNP r25191g/a and risk of KBD (OR 1·153; 95 % CI 0·533, 2·496). In addition, when the data were adjusted for potential confounding factors (adjusted for age and sex) also, no significant association was found (data not shown).
Table 1 Single nucleotide polymorphism (SNP) r25191g/a genotype and allele frequencies in patients, controls and other populations and risk of developing Kashin-Beck disease (KBD)
(No. of subjects and percentages; odds ratios and 95 % confidence intervals)
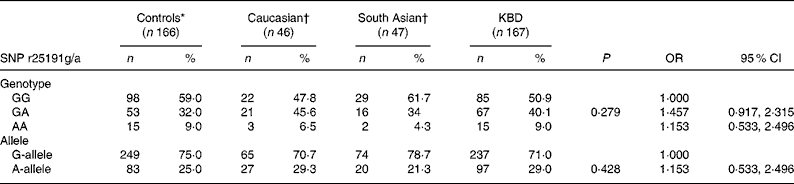
* Control groups in the present study.
† Data reported by Calvo Alfonso et al. (Reference Alfonso, Nianqing and Jason9).
Furthermore, the distributions of the SEPP1 genotype and allele in the control groups were compared with that in other population groups (Table 1). Interestingly, the genotype distribution for the homozygous GG and AA genotypes for the Chinese population were similar to those reported for the Caucasian and South Asian populations(Reference Volker10). In all ethnic groups, the frequency of the AA genotype was rare compared with the GG homozygote or GA heterozygote. However, the distribution of the heterozygous GA genotype was notably different in the Caucasian group than in the Chinese control group (46 v. 32 %). In addition, a decreased frequency of the SEPP1 gene A allele and an increased frequency of G allele were observed in the Chinese population group than in the Caucasian group.
Quantitative real-time PCR
The SEPP1 mRNA expression in the whole blood was lower in the KBD group than in the control group (0·149-fold), but it was much higher in articular cartilage tissue in the KBD group than in that in the control group (4·525-fold). The results indicate that differences were highly significant (P < 0·001 and P = 0·012, respectively) in both whole blood and articular cartilage tissue between the two groups (Fig. 1).

Fig. 1 The selenoprotein P (SEPP1) mRNA expression in whole blood and articular cartilage tissue. The SEPP1 mRNA expression in whole blood was lower in the Kashin-Beck disease (KBD) group (![]() ) than in the control group (
) than in the control group (![]() ) (0·149-fold), but it was much higher in articular cartilage tissue of the KBD group than in that of the control group (4·525-fold). Mean value was significantly different from that of the control group: *P = 0·012, **P < 0·001.
) (0·149-fold), but it was much higher in articular cartilage tissue of the KBD group than in that of the control group (4·525-fold). Mean value was significantly different from that of the control group: *P = 0·012, **P < 0·001.
Discussion
Genotype analysis
SEPP1, found predominately in the plasma, is also expressed in other tissues and is presumably secreted by them(Reference Sasakura and Suzuki11). Some authors propose that it might also act as an antioxidant and heavy-metal chelator in the extracellular matrix(Reference Al-Taie, Seufert and Mörk12). With the identification of a complex repeat structure within the SEPP1 promoter and analysis of this regulatory DNA sequence, a complex repeat structure within the SEPP1 promoter may be of functional relevance to SEPP1 gene expression(Reference Peters, Chatterjee and Hayes13).
Epidemiological studies have shown that the distribution of KBD was identical to that found in Se-deficient regions in China, and that children in KBD-endemic areas who are at the risk of developing the disease come under the nutritional status of Se deficiency with lower Se in blood, urine and hair compared with children free from the disease in endemic areas(Reference Saadat14). Therefore, we aimed to clarify whether polymorphisms of SEPP1, a special selenoprotein, were correlated with KBD risk. According to the present results, we were unable to confirm this possibility and found no correlation between SNP r25191g/a in SEPP1 gene and the risk of developing KBD among the Chinese population. As this report is the first to investigate the association of SNP r25191g/a in SEPP1 gene with KBD and the number of subjects in the present is limited, further study is necessary to draw a conclusion.
In the present study, we compared the distribution of our control study group with that of other populations to identify whether the distribution of the SNP r25191g/a in SEPP1 genes is different in other healthy population groups (Table 1). The distribution of the GA and AA genotypes at position SNP r25191g/a in SEPP1 genes in the present control study group was different from that found in a Caucasian group, and was similar to that observed in a South Asian population. Although this result should be considered with caution as the sample size of the Caucasian group was very small, the data highlight the possible variability of protein gene frequencies in different population groups and hence influence the disease outcome. Further study is necessary as the distribution of the AG of SNP r25191g/a was notably different between the control groups of Chinese and some other populations. At the same time, some genetic polymorphisms associated with an increased risk of multi-factorial diseases occur only among particular ethnic populations, and other co-factors such as environmental factors, viral load, lifestyle factors and other transmitted diseases have also been established as risk co-factors for KBD. It is recommended that the same study be carried out in other ethnic populations with KBD.
Selenoprotein P mRNA expression
The results of real-time PCR study revealed a lower expression of SEPP1 mRNA in whole blood in KBD patients than in that in the healthy controls, with a higher expression in articular cartilage tissue. SEPP1 is a Se-rich plasma protein that supplies Se to the brain, testis, liver and other tissues. On account that SEPP1 is the major plasma selenoprotein, the synthesis of SEPP1 is a priority compared with that of glutathione peroxidase in supplementary Se; SEPP1 has ten selenocysteine, which may be more important in storage, transport and maintenance of Se homeostasis, so the concentration of plasma SEPP1 can better reflect the status of human Se(Reference Fujii, Saijoh and Sumino15). In the present study, the expression of mRNA in whole blood decreased in the KBD group than in that in the control group (0·149-fold, P < 0·001) (Fig. 1), indicating that the KBD patients were in a condition of Se deficiency. Under the condition of Se deficiency, GSH metabolism is affected and glutathione peroxidase activity decreases, which will increase the oxidisation to damage bone cells and articular cartilage tissue(Reference Jing, Zhao and Fu16).
The mRNA expression of lesioned cartilage tissue increased in the KBD group (4·525-fold, P = 0·012) (Fig. 1), and this result makes sense. SEPP1 binds to − 60 % of the Se in the plasma(Reference Moschos17). SEPP1 is thought to mediate two important functions: (a) the protection of tissues against oxidative stress and (b) the transport of Se in serum and possible intracellular binding of Se(Reference Artl, Mostert and Oubrahim18). Therefore, the SEPP1 in the whole blood could be transported to the impaired cartilage tissue in order to play the role of an antioxidant. As described previously, KBD is a chronic osteoarthropathy with articular cartilage degeneration and necrosis. Thus, the significant increase in SEPP1 mRNA levels in impaired articular tissues of KBD patients may be a reflection of cartilage cell damage repair. In this process, some specific indistinct cytokines and receptors may play a crucial role. Several studies have indicated that SEPP1 mRNA and protein expression can be influenced by different cytokines such as IL-1b, TNF-a, transforming growth factor-b1 and interferon-g(Reference Mostert, Dreher and Kohrle19, Reference Hesse-Bahr, Dreher and Kohrle20). So, additional multicentre follow-up studies such as SEPP1 expression detection are necessary to confirm the difference in SEPP1 mRNA level in different tissues between KBD patients and controls.
The present study found no correlation between SNP r25191g/a in SEPP1 gene and the risk of developing KBD among the Han Chinese population. However, the frequency of the rare high-producing allele AG of SNP r25191g/a was significantly lower in the Chinese population than in the Caucasian group. SEPP1 mRNA in the KBD group was down-regulated in blood and up-regulated in articular cartilage tissue (0·149-fold and 4·525-fold, respectively), and the difference was statistically significant (P < 0·001 and P = 0·012, respectively). Considering the interaction of SEPP1 mRNA expression with some specific indistinct cytokines and receptors, a clear understanding of the underlying association between the pathogenesis of KBD and SEPP1 is required.
Acknowledgements
The present work was supported by the National Natural Science Foundation of China, No. 30671820. All the authors declare that there is no conflict of interest. W. Y. S. carried out the studies and drafted the manuscript. W. X. and X. Z. Z. participated in the study design and helped to draft the manuscript. R. X. S., X. H. D. and J. H. participated in the study design and performed the statistical analysis. Y. M. X. conceived the study and participated in its design, and helped to draft the manuscript. We thank Y. X. Yu and W. Pang for the sample collection.




