Linear growth falters dramatically in developing countries between early infancy and the end of the second year( Reference Victora, de Onis and Hallal 1 ). Variable factors are responsible for the decline in growth indicators, among them is complementary feeding (CF) that may be inadequate in quantity and/or quality relative to children's energy and nutrient needs, or that may be the conduit of infectious agents and toxins( Reference Brown, Zeitlin and Peterson 2 – Reference Imdad, Yakoob and Bhutta 6 ). Given the long-term consequences of poor linear growth in the first two years, it is imperative that interventions are implemented to break the cycle that leads to successive generations of short adults with increased risk of CVD and low economic productivity, among other problems( Reference Victora, Adair and Fall 7 ).
Various agencies attempting to address feeding-related causes of suboptimal growth in developing countries have defined a set of indicators for assessing infant and young child feeding (IYCF) practices( 8 ). The indicators could be used in quantifying relationships between key dimensions of feeding and growth among children below 2 years of age. A recent analysis of Demographic and Health Surveys (DHS) data from forty-six countries found population-level associations between IYCF indicators and levels of malnutrition. Compared with Latin America, Asia and Africa had higher prevalences of undernutrition and lower prevalences of exclusive breast-feeding, of adequate dietary diversity and overall dietary quality( Reference Lutter, Daelmans and de Onis 9 ). With specific reference to the age range of CF, the proportion of children aged 6–23 months who received a minimum acceptable diet was 16 % in Africa and 26 % in Asia compared with 43 % in the Americas.
Over the years different approaches have been used to create indicators of dietary diversity and study its association with child undernutrition. For example, prior to the publication of the IYCF indicators( 8 ), Ruel and Menon (2002)( Reference Ruel and Menon 10 ) created a child feeding index using 24 h and 7d recall data in seven Latin American surveys. They found significant associations between CF practices and height-for-age Z-scores. Similarly, Arimond and Ruel (2004)( Reference Arimond and Ruel 4 ) analysing DHS data from eleven countries created a dietary diversity score based on seven food groups and found that it was positively associated with height-for-age in nine of the countries. In a rural Bangladesh study, Rah et al. (2010)( Reference Rah, Akhter and Semba 11 ) constructed a dietary diversity score by summing the number of days each of nine food groups was consumed in the previous week. They found that reduced dietary diversity was a strong predictor of stunting. The WHO IYCF indicators have more recently been studied in DHS data from fourteen low-income countries( Reference Marriott, White and Hadden 12 ). The data from all fourteen countries were pooled and analyses were performed for disaggregated age groups for each of the indicators. The risk of both stunting and underweight was reduced with consumption of a minimum acceptable diet and minimum dietary diversity, while minimum meal frequency was associated with a lower risk of underweight only. Similar analyses for Cambodia (DHS 2005) had been carried out by the same authors in 2010, but examining a single composite variable for compliance with all age-relevant feeding indicators( Reference Marriott, White and Hadden 13 ).
The present analysis will add to the body of literature on child feeding practices in association with linear growth in the age period 6–23 months. Using twenty-one DHS data sets, the present analyses focus on a sub-set of the WHO IYCF indicators. We compare the relative strengths of the association between length-for-age and three CF indicators.
Methods
The current analysis uses data from Phase V DHS carried out between 2003 and 2008, for which CF indicator data and stunting prevalence estimates were available. Twenty-one data sets had the required data for the proposed analysis: four from Asia (Bangladesh, Cambodia, India and Nepal); twelve from Africa (Benin, Democratic Republic of Congo (DRC), Egypt, Ethiopia, Ghana, Liberia, Mali, Namibia, Niger, Uganda, Zambia and Zimbabwe); four from the Americas (Dominican Republic, Haiti, Honduras and Peru); and one from Europe (Azerbaijan). We always used the latest versions of the data sets available on the DHS website. Since the focus of the analysis is CF, only data for children aged 6 to 23 months were used. Several variables (age, sex, length, mother's height, mother's age, mother's education, wealth index and rural/urban residence) expected to be related with growth status in the age range of interest were extracted from the DHS data files, as were descriptors of CF from reported 24 h consumption by children of specific foods and liquids, and their breast-feeding (BF) status.
For the analysis, we recalculated children's exact age in months (number of days from day of birth to day of measurement divided by 30·4375). Consequently, the sample sizes may vary somewhat from those presented in other reports using the same DHS data, where age calculation by the century month code classifies some children in higher age categories of completed months if they were born late in a given month and are measured early in another month.
A binary code was created for current BF status. We included both breast-fed and non-breast-fed children when deriving the IYCF indicators. Binary variables indicating whether a child's CF met the requirements of minimum dietary diversity and minimum meal frequency were derived in accordance with the IYCF indicator definitions( 8 ). However, whereas according to the standard indicator definitions at least two milk feeds are required for milk to count towards minimum acceptable diet for non-breast-fed children, in the present analysis any milk feed was counted because the number of feeds is not recorded in DHS Phase V. For Bangladesh, we recovered a variable reporting consumption of goat's milk and included it in the diversity count as a dairy food.
Attained linear growth, namely length-for-age Z-score (LAZ) based on the WHO child growth standards( 14 ), was calculated for use as the primary outcome for the analyses presented in this paper. Extreme LAZ values, i.e. beyond the interval −6 and +6, were flagged and excluded following standard practice when calculating Z-scores in the WHO Anthro software( 15 ).
Descriptive summary statistics for each country are based on weighted analysis using DHS sampling weights. However, for assessing associations between linear growth and dietary factors at child level, the analysis was un-weighted.
ANOVA was carried out separately for each country considering that, for example, wealth quintiles in Azerbaijan and Mali are not comparable in absolute terms. LAZ was treated as dependent variable and the IYCF indicators – minimum dietary diversity, minimum meal frequency and current BF – as independent variables, adjusted by the following covariates: child's age and sex; mother's height, age and education (none, primary, secondary and higher); wealth index quintiles (as defined in respective DHS data sets( Reference Rutstein and Kiersten 16 )); and rural/urban residence. The CF indicator of minimum acceptable diet is a composite of minimum dietary diversity and minimum meal frequency. Therefore, a set of models was evaluated to compare the effects of the composite variable v. the disaggregated components (please note that the term ‘effects’ refers to the difference in adjusted means associated with different levels of the factor of interest in ANOVA). Possible interactions between factors of interest also were investigated. Covariate age was categorized into four groups: 6–8, 9–11, 12–17 and 18–23 completed months, and interactions with minimum dietary diversity and current BF status were examined.
Separate ANOVA models were run for each country, beginning with the full list of CF factors, interactions of interest and the covariates described above. Model selection proceeded by the backward stepwise approach. Non-significant dietary factors and interactions (P ≥ 0·05) were eliminated, except where a non-significant factor was involved in a significant interaction with another variable. All covariates were retained in the models regardless of their statistical significance.
For a detailed exploration of interrelationships between diet diversity and increased stunting with age, each sample was stratified into three length-for-age categories: LAZ<−2, LAZ = −2 to <−1 and LAZ ≥ −1, and age-related patterns were examined.
Analyses were done using the SAS statistical software package version 9·2 and the S-Plus statistical software package version 8·2. Statistical significance at P < 0·05 is reported.
Results
Table 1 presents basic descriptive information for each of the twenty-one countries, with sample sizes ranging from 646 to 14820. Five countries had at least half of the sample receiving the minimum dietary diversity, namely Azerbaijan (53·1 %), Egypt (56·8%), Honduras (67·3 %), Dominican Republic (74·4 %) and Peru (80·6 %). At the opposite extreme were Ethiopia (4·0 %), Niger (5·5 %), DRC (11·4 %), India (12·3 %) and Mali (16·3 %). Although minimum meal frequency was generally better assured than dietary diversity, less than half of the sample in Azerbaijan, Dominican Republic, Haiti, India and in all sub-Saharan African countries achieved minimum meal frequency. Considering the composite measure encompassing dietary diversity and meal frequency, only in Peru did the majority of the sample (66·0 %) receive a minimum acceptable diet, while coverage in the rest ranged from 42·0 % in Honduras to just 2·8 % in Ethiopia, 3·1 % in DRC and 3·2 % in Niger.
Table 1 Description of feeding indicators, covariates and stunting among children 6–23 months of age; secondary analysis of Phase V Demographic and Health Surveys data (2003–2008)Footnote †
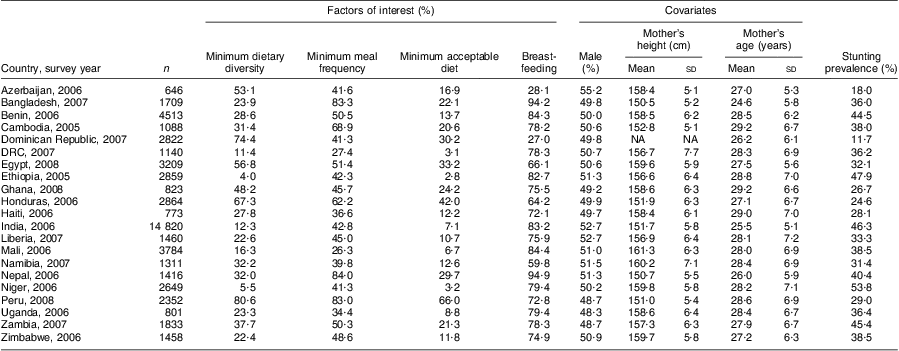
DRC, Democratic Republic of Congo; NA, not available.
† Weighted analysis using Demographic and Health Survey sampling weights.
Proportions of children currently breast-fed ranged between 28 % and 95 % overall, with marked age-related differences, as expected (data not shown). For children aged 6–18 months, all but four countries (Azerbaijan, Dominican Republic, Honduras and Namibia) had BF rates of over 80 %. At 18–23 months, less than one-sixth of the samples in Azerbaijan and Dominican Republic, and about one-third in Egypt, Namibia and Zimbabwe, were breast-fed, as were 50 % or less in Ghana, Honduras, Haiti, Liberia, Peru and Zambia, compared with majorities in the remaining ten countries, including 89·2 % in Bangladesh and 89·9 % in Nepal. The gender distribution in the samples was about even with the most variant being Azerbaijan (55·2 % male) and Uganda (48·3 % male). Maternal heights segregated into two distinct groups, with six countries averaging 151–153 cm and the remaining countries clustering in the 157–161 cm bandwidth. The Dominican Republic data set was missing mother's height. Stunting rates ranged from 11·7 % (Dominican Republic) to 53·8 % (Niger). There was a clear negative correlation between minimum dietary diversity and stunting (simple linear regression slope −0·35, R 2 = 58 %). Countries were clustered in two blocks: those with minimum dietary diversity rates below 40 % and stunting rates of 30 % or above (fifteen countries), and those with minimum dietary diversity above 40 % and stunting rates below 30 %. The exception was Egypt, with stunting rate of 32·1 % and minimum dietary diversity rate of 56·8 %.
Minimum acceptable diet yielded mostly non-significant results in the alternative models where it was tested in place of dietary diversity and meal frequency as separate variables (data not shown). Significant effect estimates were found in six countries: Bangladesh (6–9 months and 18–23 months), Honduras (18–23 months), and India, Cambodia, Nepal and Zambia (6–23 months). In all countries but Zambia, minimum dietary diversity was associated with greater differences in LAZ compared with minimum acceptable diet. We therefore used the two separate indicators of dietary diversity and meal frequency in all subsequent analyses.
Table 2 summarizes dietary factors and interactions retained in the final models for each country. Minimum dietary diversity and current BF each had significant effect estimates in twelve out of twenty-one countries. Conversely, minimum meal frequency had statistically significant effect estimates in only four countries (0·08 LAZ in India, 0·19 in Zambia, 0·24 in Ethiopia and −0·25 in Zimbabwe). The interaction between age and minimum dietary diversity was significant in seven countries (Bangladesh, DRC, Egypt, Ghana, Honduras, Niger and Uganda), between age and current BF in three countries (India, Liberia and Uganda), and between minimum dietary diversity and current BF in two countries (DRC and Peru).
Table 2 Dietary factors associated with length-for-age among children 6–23 months of age based on ANOVA adjusted by demographic covariatesFootnote †; secondary analysis of Phase V Demographic and Health Surveys data (2003–2008)
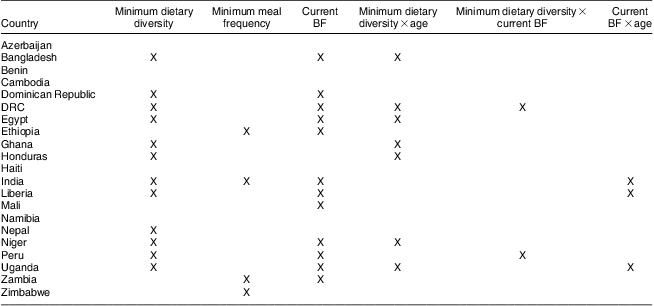
BF, breast-feeding; DRC, Democratic Republic of Congo.
† Covariates included in the ANOVA models: child age and sex; mother's height, age and education (none, primary, secondary and higher); wealth index quintiles (as defined in respective Demographic and Health Survey data sets); and rural/urban residence.
The change in LAZ through age categories was consistent across countries: the highest average LAZ values were observed in the youngest category (6–9 months), followed by systematic downward trends to the end of the second year (Table 3). The countries where mean LAZ originated close to the standard median at 6–9 months were Ghana (+0·03), Dominican Republic (−0·18), DRC (−0·38) and Mali (−0·40). On the other hand, the samples in Bangladesh, Benin and Nepal already had at 6–9 months mean LAZ<−1. By the end of the second year, the average child in Benin, Cambodia, Ethiopia, India, Nepal, Niger and Zambia was stunted (mean LAZ<−2).
Table 3 Length-for-age Z-score by age group; secondary analysis of Phase V Demographic and Health Surveys data (2003–2008)

DRC, Democratic Republic of Congo.
Dietary diversity was the CF factor most consistently associated with length-for-age as highlighted in Table 4, which presents dietary diversity effect estimates as mean differences in LAZ between children receiving the minimum diet diversity and those who did not, adjusted by the standard set of covariates described earlier. Where the interaction with age or BF was significant, mean differences are presented by respective categories. ANOVA results in nine countries revealed no significant difference in mean LAZ by minimum dietary diversity group. In eleven countries, the association was positive and significant, with effect estimates ranging from 0·16 to 1·40 LAZ in favour of increased dietary diversity. For Egypt, higher diversity was negatively associated with LAZ (−0·31) in the 12–18 months age group while for 6–9-month-olds, the association was positive (0·42). Significant interactions with BF were observed in DRC and Peru, showing that dietary diversity was positively associated with LAZ only in children who were no longer breast-fed.
Table 4 Detailed comparisonsFootnote † of length-for-age Z-scores (LAZ) between minimum dietary diversity groups by age category; secondary analysis of Phase V Demographic and Health Surveys data (2003–2008)

DRC, Democratic Republic of Congo.
* Significant at 5 % level.
† Estimated difference, 95 % confidence interval and sample sizes (n yes, n no) based on ANOVA models as presented in Table 2.
‡ Minimum dietary diversity main effect estimate (difference in LAZ found in the whole age interval) when interaction with age was not statistically significant.
The analysis of interrelationships between diet diversity and length-for-age categories by age (data not shown) showed that, in most countries, the children in the lowest LAZ category were less likely than the others to be receiving a diet with the minimum diversity. These results are consistent with those summarized in Table 4. Examining age-related changes in median dietary diversity, it was remarkable that the countries with consistently low diversity experienced the largest declines in LAZ. Niger, for example, had the largest decline in length-for-age (1·9 Z-scores) from the youngest through to the oldest age group in the sample. Correspondingly, the proportions with LAZ<−2 increased from 25 % in the group aged 6–9 months to 70 % at 18–23 months, with a dietary diversity median constant at 1 in this LAZ stratum. In the Mali sample, the average child aged 6–9 months received no complementary feeds (median dietary diversity = 0) and for the older age groups, only one food group (median dietary diversity = 1). Accordingly, length-for-age declined by 1·6 Z-scores, from −0·4 at 6–9 months to −2·0 at 18–23 months. Similar patterns were observed in DRC and Ethiopia where median dietary diversity increased from 1 at 6–9 months to 2 at 18–23 months, and length-for-age declined by 1·3 or larger Z-scores. Conversely, median dietary diversity in Peru and the Dominican Republic increased from 3–4 at 6–9 months to 5 at 18–23 months and associated declines in length-for-age were 0·7 and 0·6 Z-scores, respectively.
Discussion
Results of the present analysis confirm a pattern that has been previously documented where children in developing countries experience systematically declining length-for-age during the CF period( Reference Victora, de Onis and Hallal 1 , Reference Shrimpton, Victora and de Onis 17 ). There is justifiable concern to intervene before the cumulative deficits in linear growth become irreversible after age 2–3 years( Reference Martorell, Khan and Schroeder 18 , Reference Stein, Barnhart and Wand 19 ) with adverse consequences in adulthood and for future generations( Reference Victora, Adair and Fall 7 ). In addition to the need to prevent/treat infections and promote continued BF, there is growing recognition that poor CF contributes to the characteristic negative growth trends observed in developing countries and needs focused attention and its own tailored interventions. To this end, indicators of CF quantity and quality were included in the IYCF indicators for assessing practices, identifying and targeting populations at risk, and monitoring and evaluating IYCF interventions( 8 ).
Among the CF indicators examined in the present analysis, dietary diversity was associated with improved linear growth more consistently than was meal frequency. Others have reported similar positive associations between dietary diversity and child growth( Reference Arimond and Ruel 4 , Reference Ruel and Menon 10 , Reference Steyn, Nel and Nantel 20 ), in some cases identifying animal-source foods as the main factor associated with improved linear growth( Reference Marquis, Habicht and Lanata 21 – Reference Krebs, Mazariegos and Tshefu 23 ). The increasing likelihood of becoming stunted between the ages of 6 and 23 months in some countries, particularly in sub-Saharan Africa, appeared to be associated with inadequate diversification of the diet. This was especially well illustrated in Mali and Niger where length-for-age dropped by >1·6 Z-scores in contrast to comparatively lower declines in the Dominican Republic and Peru, where CF indicators were a lot more favourable and dietary diversity increased with age. For the indicator that combines dietary diversity and meal frequency, the proportions of children receiving a minimum acceptable diet were very low, pointing to inadequacies in both quality and quantity of CF in the majority of the countries studied. In Zimbabwe, only minimum meal frequency compliance was associated with attained linear growth after adjusting for the covariates. However, this association was negative for reasons that cannot be explained within the scope of available data.
In six of the countries included in the present analysis (Azerbaijan, Benin, Cambodia, Haiti, Mali and Namibia), none of the CF indicators was significantly associated with attained linear growth in models adjusting for maternal and household-level covariates. We did not find any published reports with comparable analysis of CF practices and their association with linear growth for Azerbaijan, Mali or Namibia.
The lack of association between feeding indicators and linear growth in Benin is not new: the analysis of DHS data by Arimond and Ruel( Reference Arimond and Ruel 4 ) also failed to find a significant association between dietary diversity and length-for-age in this country. On the other hand, exposure to aflatoxins (ingested through complementary foods) has been associated with stunting( Reference Gong, Cardwell and Hounsa 24 ), and another study in Benin described high intestinal parasite loads in pre-school children who also received diets that were low in energy and Fe and that contained high amounts of fibre and phytate( Reference Dossa, Ategbo and de Koning 25 ). As happened for Benin, dietary diversity had a non-significant association with length-for-age in models adjusted for a selection of covariates in Arimond and Ruel's analysis of Haiti DHS 2000 data( Reference Arimond and Ruel 4 ). For Zimbabwe (DHS 1999), the same authors found an effect estimate of 0·59 LAZ for children in the top compared with the lowest tertile of dietary diversity( Reference Arimond and Ruel 4 ). Two recent studies from Zimbabwe have reported that CF diets are low in diversity and energy density( Reference Paul, Muti and Chasekwa 26 ), and that household factors contribute importantly to poor feeding, for example beliefs against children consuming animal-source foods, legumes, fruits and vegetables, as well as competing demands on mothers’ time that limit their ability to feed their children adequately( Reference Paul, Muti and Khalfan 27 ). Analysing the survey data for Cambodia also used for the present paper (DHS 2005), Marriott et al. found a lower risk of stunting among the children aged 6 and 11 months whose feeding complied with all the age-relevant indicators( Reference Marriott, White and Hadden 13 ).
We acknowledge that the present analysis has the typical limitations of using cross-sectional data that were collected for other purposes than to study the relationship between growth and CF. Attained length is the result of cumulative exposures to multiple factors that cannot be captured in the complementary diet consumed in a single day, even with adjustment for important household variables. Additionally, creating dichotomous variables reduces precision relative to the original measurement scale of the CF variables and entails loss of power to detect significant associations between them and growth outcomes.
These results nevertheless provide a few potentially useful insights with regard to programmes/interventions addressing CF. In the first place, because stunted growth is the result of multifaceted influences, expectations about improvements in response to single interventions should be modest. Overall, age-related declines in LAZ were larger than the positive effect estimates associated with improved CF. Two variants of food assistance programmes in Haiti with preventive interventions (including CF) among 6–23-month-olds and curative interventions (food supplements) targeting underweight 6–59-month-olds had effect estimates of 0·34 and 0·18 LAZ, respectively( Reference Ruel, Menon and Habicht 28 , Reference Donegan, Maluccio and Myers 29 ). Improved CF is a preventive intervention whose timeliness makes it attractive as a measure to mitigate decline in length-for-age before the cumulative deficits of stunting become irreversible after age 2 years. In the present analysis, positive effect estimates associated with minimum dietary diversity ranged widely from 0·2 to 1·4 LAZ. The largest estimated differences were in two countries (DRC and Uganda) where only small proportions of the sample received minimum dietary diversity and the standard deviations around the mean LAZ were large. This positive deviance may be due to additional health-promoting practices that characterize households that feed varied diets to their toddlers.
Our results and others’ show that dietary diversification has potential to improve linear growth. However, some of the benefits may be annulled if the foods included in the diversified diet carry infectious agents associated with poor hygiene( Reference Humphrey 5 , Reference Motarjemi, Käferstein and Moy 30 ). In regions where weaning foods are likely conduits of aflatoxins, which suppress linear growth, it is critical to take necessary measures to keep complementary foods safe( Reference Gong, Hounsa and Egal 31 , Reference Khlangwiset, Shephard and Wu 32 ).
Although the selection of foods available in the countries we studied must be quite diverse, using four food groups to define minimum dietary diversity appears to capture diet quality in a manner that makes it comparable across the world's developing regions. This gives credence to the use of this indicator for multi-country comparisons, especially when associated child growth is a question of interest. In this regard, minimum acceptable diet may be a useful descriptor of CF quantity and quality but perhaps its derivation leads to a loss of precision in relation to its likely contribution to growth outcomes.
Acknowledgements
Sources of funding: This analysis was conducted at the WHO as part of Promoting Healthy Growth and Preventing Childhood Stunting, a project funded by the Bill and Melinda Gates Foundation. The funder had no role in the design, analysis or writing of this article. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of the WHO or of Boston College. Conflicts of interest: No author has a personal or financial conflict of interest. Ethics: Ethical approval was not required to conduct the secondary analysis of DHS data. Authors’ contributions: The analysis plan was conceptualized by A.W.O and E.B., and reviewed and developed further by M.d.O, M.d.C.C. and C.G. E.B. analysed the data and wrote up the methods section, A.W.O. drafted the manuscript that was critically reviewed and refined by all authors. Acknowledgements: Special thanks are extended to Kathryn G. Dewey, Kim F. Michaelsen, Chessa Lutter and Bernadette Daelmans for reviewing the analysis plan and manuscript, and to Monica Kothari and Noureddine Abderrahim for providing DHS data codes and results from previous analyses of the DHS data.






