Postpartum depression (PPD) affects up to 20 % of women within the first 12 months of parturition(Reference Gaynes, Gavin and Meltzer-Brody1). Similar to major depressive disorder (MDD), symptoms may include sadness, anhedonia, feeling overwhelmed, mood lability, irritability, sleep and appetite disturbance, and guilt(Reference Gaynes, Gavin and Meltzer-Brody1–Reference Steward and Vigod3). Peripartum (antepartum and postpartum) depression can have negative consequences for both the mother and developing child, and hence diagnosis and appropriate treatment should be of paramount public health priority(Reference Brockington, Butterworth and Glangeaud-Freudenthal4). Nutritional measures have been utilized as both treatment interventions and as possible markers of depression in the peripartum period, and pregnant women are susceptible to the effects of nutrient deficiencies due to increased nutritional demands from the developing fetus(Reference Bodnar and Wisner5, Reference Ellsworth-Bowers and Corwin6). Standard prenatal vitamins may be inadequate for the requirements of specific nutrients, such as vitamin D(Reference Bodnar, Simhan and Powers7).
The term ‘vitamin D’ encompasses a family of secosteroid hormones produced after exposure to sunlight (i.e. via UVB radiation) in fungi (ergocalciferol; vitamin D2) and in animal tissue (cholecalciferol; vitamin D3). Cholecalciferol is produced endogenously in human skin after exposure to UVB radiation, especially during the summer. Hence, sunlight is critical to vitamin D status given the limited availability of ergocalciferol and cholecalciferol from dietary sources(Reference Shin, Choi and Longtin8). Ergocalciferol and cholecalciferol are metabolized in the liver to form 25-hydroxyvitamin D (25(OH)D), the predominant form of vitamin D in the blood(Reference Miller and Peters9). In addition to its importance in bone health(Reference Miller and Peters9), 25(OH)D may function in reproduction(Reference Grundmann and von Versen-Höynck10, Reference Mumford, Garbose and Kim11) and in the promotion of immune health(Reference Shin, Choi and Longtin8, Reference Brannon12). Exposure to sunlight and UVB radiation are important considerations when evaluating for 25(OH)D. For example, lower concentrations are expected in the winter months, which are characterized by shorter days and therefore decreased exposure to sunlight(Reference Holick, Binkley and Bischoff-Ferrari13). Another important consideration is skin pigmentation because melanin differentially absorbs UVB. Hence, African Americans are at higher risk for vitamin D deficiency compared with Caucasian Americans(Reference Bodnar, Simhan and Powers7, Reference Harris14). Recent research has implicated low 25(OH)D as a possible cause of depressive symptoms(Reference Hoang, Defina and Willis15), which is more pronounced in women than men(Reference Gur, Genc and Eskicioglu16–Reference Polak, Houghton and Reeder19). This finding continues to be investigated, and a causal link remains to be elucidated.
As stated by the Institute of Medicine, 25(OH)D sufficiency is defined as ≥ 20 ng/ml (≥50 nmol/l) and deficiency is defined as <12 ng/ml (<30 nmol/l); the range between, 12–20 ng/ml (30–50 nmol/l), is defined as potential risk for inadequacy. Greater than 30 ng/ml (>75 nmol/l) is not associated with improved benefit(20). Conversely, the Endocrine Society defines 25(OH)D deficiency as <20 ng/ml (<50 nmol/l), insufficiency as 21–29 ng/ml and sufficiency as 30–100 ng/ml(Reference Holick, Binkley and Bischoff-Ferrari13). Although these ranges differ slightly, both organizations agree that <20 ng/ml is potentially inadequate or worse, and the more stringent criteria by the Endocrine Society recommend a target minimum concentration of 30 ng/ml(Reference Holick, Binkley and Bischoff-Ferrari13).
Several studies have demonstrated correlations of low 25(OH)D concentration in early pregnancy with increased depressive symptomatology later in pregnancy(Reference Brandenbarg, Vrijkotte and Goedhart21–Reference Cunha Figueiredo, Trujillo and Freitas-Vilela23). The present commentary encompasses the findings of currently published literature regarding 25(OH)D deficiency during pregnancy and depressive symptoms in the postpartum period.
Methods
A literature search was performed using PubMed, PsycINFO and Web of Science. Keywords included combinations of ‘pregnancy’ or ‘postpartum’ with ‘depression’ and ‘vitamin D’. Studies were considered if published in peer-reviewed, English-language journals, if a serum 25(OH)D concentration was obtained during pregnancy (prior to the day of parturition), and a validated assessment for depressive symptoms was utilized during the postpartum period. Articles were excluded if written as a review article or case report, not focused on human research, or if oral administration of vitamin D was not verified by a corresponding serum concentration. While over 150 articles were initially identified, only five were relevant after excluding duplicates and applying inclusion and exclusion criteria. Data extraction from each study included the author, year of publication, sample size, study description, time of serum collection, scale of depressive symptom assessment and a brief description of reported outcomes (Table 1). The studies are organized by chronology, then alphabetically.
Table 1 25-Hydroxyvitamin D deficiency and postpartum depressive symptoms
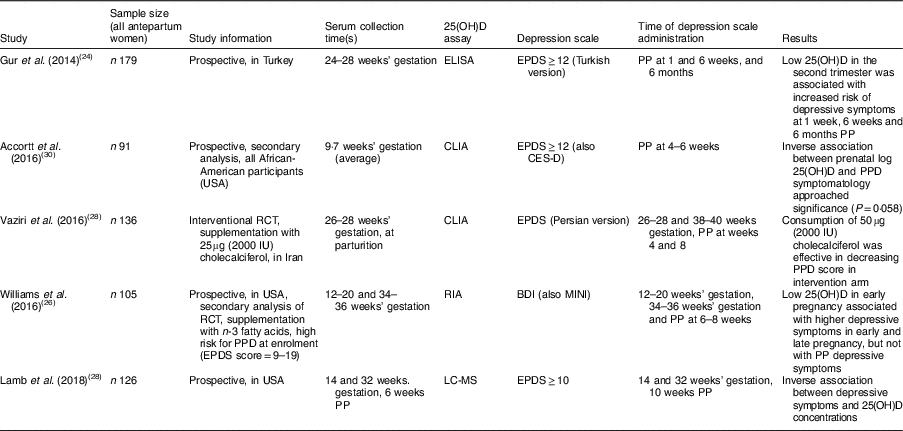
25(OH)D, 25-hydroxyvitamin D; RCT, randomized controlled trial; PPD, postpartum depression; EPDS, Edinburgh Postnatal Depression Scale; CLIA, chemiluminescence immunoassay; CES-D, Center for Epidemiological Studies Depression Scale; BDI, Beck Depression Inventory; MINI, Mini International Neuropsychiatric Interview; PP, postpartum.
Results
As delineated in Table 1, four of the five studies utilized the Edinburgh Postnatal Depression Scale (EPDS)(Reference Cox, Holden and Sagovsky24) for evaluation of depressive symptoms, and the study by Williams et al. used both the Beck Depression Inventory (BDI)(Reference Beck, Ward and Mendelson25) and the clinician-administered Mini International Neuropsychiatric Interview (MINI)(Reference Williams, Romero and Clinton26). All studies used the Endocrine Society’s definition of 25(OH)D deficiency, <20 ng/ml(Reference Holick, Binkley and Bischoff-Ferrari13).
Gur and colleagues recruited 179 medically healthy pregnant women from a university hospital setting in Turkey(Reference Gur, Gokduman and Turan27). They measured serum 25(OH)D between 24 and 28 weeks’ gestation and at 6 months postpartum. 25(OH)D was defined as severely deficient if <10 ng/ml and mildly deficient if <20 ng/ml; the prevalence in the antepartum women was 11·0 and 40·3 %, respectively. These women were assessed by EPDS (Turkish version) at 1 week, 6 weeks and 6 months postpartum. Depression (defined as EPDS≥12) ranged from 21·6 to 23·7 %. Lower maternal 25(OH)D at 24–28 weeks’ gestation was associated with higher levels of depressive symptoms at 1 week, 6 weeks and 6 months postpartum. The study did not control for other risk factors, such as personal or family history of MDD. Additionally, it excluded women ‘prone’ to PPD for reasons including unmarried status, unplanned pregnancy, low socio-economic status, medical or psychiatric illness at time of enrolment, and complications at time of delivery(Reference Gur, Gokduman and Turan27).
Vaziri et al. performed an interventional randomized controlled trial in Iran(Reference Vaziri, Nasiri and Tavana28). The participants provided serum for 25(OH)D determination at 26–28 weeks’ gestation (baseline) and within the first 24 h postpartum. EPDS was obtained at four time points: 26–28 and 38–40 weeks’ gestation; and at 4 and 8 weeks postpartum. The women were randomized to receive supplementation of 50µg (2000IU) cholecalciferol or placebo, and baseline EPDS was not different between the two groups. At baseline, 72·8 % of the women were 25(OH)D deficient (<20 ng/ml); additionally, 9·8 % of the women were categorized as insufficient (21–29 ng/ml). In the treatment arm, the average 25(OH)D serum concentration increased by about 4·6 ng/ml over approximately 12 weeks of treatment, whereas in the placebo arm concentration remained constant (near 12 ng/ml). EPDS scores were significantly lower at all three subsequent time points in the treatment arm. Limitations of the study include: exclusion of women with EPDS>13 at first assessment (i.e. women with elevated depressive symptoms); and the cholecalciferol intervention required self-administration, which does not ensure daily supplementation(Reference Vaziri, Nasiri and Tavana28).
Lamb et al. recruited 126 pregnant women from a large urban medical centre from their prenatal appointments(Reference Lamb, Lutenbacher and Wallston29). The EPDS and serum for 25(OH)D determination were gathered at 14 and 32 weeks’ gestation. In the postpartum, serum was collected at 6 weeks and EPDS at 10 weeks. The authors concluded that the EPDS was consistent over time in their sample and elevated depressive symptoms (defined as EPDS score ≥10) was inversely correlated with 25(OH)D concentrations at all three time points. At 14 weeks’ gestation, 25(OH)D deficiency (<20ng/ml) was determined in 21·6 % of their study population; this deficiency reduced to 12·5 % by the postpartum period. An explanation for this reduction may have been the initiation of prenatal vitamins containing vitamin D. Interestingly, the authors also determined lower 25(OH)D concentrations in two populations: (i) women requiring a caesarean delivery; and (ii) women with postpartum haemorrhage. Limitations of the study include an attrition rate of 30 % by the last time point and missing 25(OH)D samples from some of the study participants(Reference Lamb, Lutenbacher and Wallston29).
Accortt and colleagues obtained serum from ninety-one African-American women at an average at 9·7 weeks’ gestation(Reference Accortt, Schetter and Peters30). They measured serum 25(OH)D as well as inflammatory cytokines. Using the cut-off of 20 ng/ml, 85 % of the women were discovered to be 25(OH)D deficient. When assessed by EPDS at 4–6 weeks postpartum, 12 % of the women had an elevated EPDS, and the authors noted that the EPDS scores were higher in overweight or obese women. Higher concentration of 25(OH)D was protective against postpartum depressive symptoms with marginal significance (P=0·058). Limitations of the study include that 50 % of the original study population (n 85) was lost to follow-up (i.e. did not complete EPDS in the postpartum period). As the study was a secondary analysis, the authors were unable to obtain a repeat measure(Reference Accortt, Schetter and Peters30).
Williams et al. performed a secondary analysis of a randomized controlled trial designed to assess for n-3 fatty acid supplementation during pregnancy as protective against postpartum depressive symptoms(Reference Williams, Romero and Clinton26). In the original study, 126 women at high risk for PPD (EPDS score 9–19, or a past history of MDD) were randomized to receive supplementation with fish oil or placebo; 105 women completed data assessments(Reference Mozurkewich, Clinton and Chilimigras31). Serum for 25(OH)D determination was obtained at 12–20 and 34–36 weeks’ gestation; 16 and 12 % of the women were deficient, respectively. The BDI was utilized to evaluate depressive symptoms, as well as the MINI for diagnostic evaluation for MDD, at four time points: 12–20, 26–28 and 34–36 weeks’ gestation; and at 6–8 weeks postpartum. 25(OH)D deficiency at 12–20 weeks’ gestation was associated with higher depressive symptoms at 34–46 weeks’ gestation. More specifically, for every 1-unit increase in 25(OH)D since 12–20 weeks’ gestation, the BDI score decreased by 0·14 units. However, no association was observed between 25(OH)D deficiency and postpartum depressive symptoms. Limitations of the study include that it was a secondary data analysis of an interventional study that was designed to reduce depressive symptoms by treatment with n-3 fatty acids, and hence the original study purpose may have confounded these results(Reference Williams, Romero and Clinton26).
Discussion
Epidemiological evidence regarding the causation of depressive symptoms by 25(OH)D deficiency is limited, but some theorize that vitamin D may play an important role in mood(Reference Hoang, Defina and Willis15). This theory may prove to be more critical in women than men(Reference Gur, Genc and Eskicioglu16–Reference Polak, Houghton and Reeder19). Ellsworth-Bowers and Corwin postulated that vitamin D impacts both the immune system and the hypothalamic–pituitary–adrenal axis to assert a regulatory role in the development in depression(Reference Ellsworth-Bowers and Corwin6). Early detection and treatment of PPD should be of high public health priority to prevent negative outcomes for both mothers and children(Reference Brockington, Butterworth and Glangeaud-Freudenthal4). The present commentary is unique in its focus on antepartum 25(OH)D assessment in concert with postpartum depressive symptomatology.
In an interventional randomized controlled trial with cholecalciferol supplementation, Vaziri et al. demonstrated improvement in both 25(OH)D serum concentration and EPDS scores by the end of pregnancy and at 2 months postpartum in mothers with 25(OH)D deficiency during the second trimester of pregnancy(Reference Vaziri, Nasiri and Tavana28). Similarly, Gur and colleagues measured 25(OH)D serum levels in women during their second trimester of pregnancy and determined increased depressive symptoms up to 6 months postpartum in 25(OH)D-deficient women(Reference Gur, Gokduman and Turan27). Most recently, Lamb et al. determined 25(OH)D deficiency during the first and third trimesters to be associated with increased depressive symptoms at 10 weeks postpartum(Reference Lamb, Lutenbacher and Wallston29). The two remaining studies reviewed here were secondary analyses and therefore were primarily designed to evaluate other hypotheses. Thus, their findings are interpreted with reservation as certain confounders were unavoidable.
Multiple studies have demonstrated that 25(OH)D deficiency during the first trimester of pregnancy is predictive of elevated depressive symptoms during the second and/or third trimesters(Reference Brandenbarg, Vrijkotte and Goedhart21–Reference Cunha Figueiredo, Trujillo and Freitas-Vilela23). In three of the studies reviewed here, the prevalence of 25(OH)D deficiency was ≥40 %(Reference Gur, Gokduman and Turan27, Reference Vaziri, Nasiri and Tavana28, Reference Accortt, Schetter and Peters30). Clinicians may be surprised to learn of the high prevalence of 25(OH)D deficiency during pregnancy, which has been demonstrated in pregnant women living in other locations with high sun exposure (Brazil and Australia)(Reference Cunha Figueiredo, Trujillo and Freitas-Vilela23, Reference Robinson, Whitehouse and Newnham32). Curiously, a recent prospective cohort study discovered that 25(OH)D>30 ng/ml was associated with increased likelihood of conception and live birth in a cohort of women with a history of multiple pregnancy losses(Reference Hoang, Defina and Willis15). One plausible explanation is that requirements of 25(OH)D during pregnancy are even higher due to the demands of the developing fetus, and the threshold of 30 ng/ml may need to be redefined for this special population.
The present commentary has limitations, including the inability to directly compare studies due to methodological differences. The EPDS, utilized in most of these studies, was initially developed as an adjunct to diagnostic evaluation. While it provides a numerical score for comparison of symptoms, it does not assess for duration or intensity of depression nor clinically diagnose MDD(Reference Cox33). The studies have populations that are inherently different, such as Turkish, Iranian and African American; hence, the findings are not generalizable. Importantly, UVB radiation from sunlight plays a critical role in 25(OH)D production(Reference Shin, Choi and Longtin8), and one would expect different amounts of sun exposure based on the locations where the studies occurred.
A recent Cochrane review did not recommend routine screening of 25(OH)D in pregnant women, expressing concern for possible adverse effects of over-supplementation(Reference De-Regil, Palacios and Lombardo34). In contrast, the Endocrine Society recommends routine supplementation with cholecalciferol during pregnancy and lactation due to increased metabolic demand and as preventive against pre-eclampsia and required caesarean delivery(Reference Holick, Binkley and Bischoff-Ferrari13). Furthermore, a recent randomized field trial found a reduction in pregnancy complications (pre-eclampsia, gestational diabetes and preterm delivery) in women supplemented with cholecalciferol(Reference Rostami, Tehrani and Simbar35).
Cholecalciferol supplementation is a safe and cost-effective intervention during pregnancy(Reference Hollis, Johnson and Hulsey36). The present author and others conclude that vitamin D should be the target of more extensive research during pregnancy and the postpartum period, as it appears to be important for both the medical and mental health of the mother and developing child(Reference Bodnar and Wisner5, Reference Ellsworth-Bowers and Corwin6, Reference Grundmann and von Versen-Höynck10, Reference Gur, Genc and Eskicioglu16). All pregnant women should be screened for 25(OH)D and recommended cholecalciferol supplementation if deficient. For every 2·5 µg (100 IU) of oral supplementation, 25(OH)D serum concentration increases by approximately 1 ng/ml(Reference Hollis, Johnson and Hulsey36), which can be used as a guide for patient dosing.
Acknowledgements
Acknowledgements: The author would like to thank Michael McCarthy, MD, PhD for his guidance in the preparation of this manuscript. Financial support: The composure of this manuscript was funded by the National Institutes of Health (grant number R25 MH101072). The National Institutes of Health had no role in the design, analysis or writing of this article. Conflict of interest: There is no conflict of interest to declare. Authorship: M.J.S. was the sole author of this manuscript. Ethics of human subject participation: Not applicable.



