The overall 5-year survival rate for childhood cancers has improved significantly over the last several decades and is currently at 84 % in developed countries(Reference Miller, Siegel and Lin1). Consequently, there has been an increased focus on the long-term health and wellness of childhood cancer survivors (CCS). CCS are at increased risk for several late-effects of treatment including CVD, obesity and secondary cancers(Reference Diller, Chow and Gurney2,Reference Oeffinger, Mertens and Sklar3) . CCS, in particular those with a history of leukaemia(Reference Barnea, Raghunathan and Friedman4), tend to gain weight during the course of treatment and remain at a higher weight into survivorship, emphasizing the need for nutrition interventions throughout the cancer care continuum(Reference Zhang and Parsons5). CCS have been shown to eat inadequate amounts of whole grains, fruits, vegetables and fibre while consuming an excess of meat and sodium(Reference Zhang, Saltzman and Kelly6–Reference Guenther, Casavale and Reedy9). This finding is mirrored in non-CCS children, who demonstrate similarly low adherence to national dietary guidelines(Reference Banfield, Liu and Davis10). In particular, both CCS and non-CCS have inadequate intakes of total vegetables, whole grains, greens and beans(Reference Zhang, Saltzman and Kelly6,Reference Banfield, Liu and Davis10) .
Several strategies to promote a healthier diet have shown promise among non-CCS including family-based multicomponent interventions(Reference Waters, de Silva-Sanigorski and Burford11). Given that CCS and non-CCS have similar dietary intake inadequacies, survivors may benefit from interventions similar to those developed for the general population. However, current CCS practices and needs must be objectively examined in order to reveal similarities and differences between CCS and non-CCS families.
The stress of treatment and the emotions associated with a diagnosis of cancer may negatively impact food choices and dietary patterns of the entire family and patient(Reference Cohen, Wakefield and Cohn12,Reference Aldhafiri, Al-Nasser and Al-Sugair13) . After completion of treatment, children and parents may struggle to break unhealthy habits from before and created during this period(Reference Zhang and Parsons5). CCS parents may demonstrate overprotective or ‘spoiling’ feeding practices, lack boundary setting, and are more likely to use monitoring and restrictive food parenting practices with their CCS(Reference Long and Marsland14–Reference Cohen, Wakefield and Fleming17). How parents of CCS translate these feeding practices and coping mechanisms into actual food preparation practices is unknown.
Nutrition intervention for CCS is a relatively new research area, and programme efficacy and best practices for this population are still under investigation(Reference Cohen, Wakefield and Cohn12,Reference Zhang, Kelly and Must18) . Understanding the similarities and differences between CCS and non-CCS family food preparation practices will help support the development of family-based nutrition resources for CCS populations. The present study describes food preparation practices of CCS and non-CCS families. Our intent is that the results of the study contribute to tailored, nutrition programming for this high-risk population.
Materials and methods
Setting and study participants
The present study used an observational, cross-sectional, mixed-methods design. Participants were parent–child dyads recruited between October 2017 and June 2018. CCS were recruited from the MD Anderson Children’s Cancer Hospital. Research staff identified eligible survivors through the MD Anderson Survivorship Network, providers and hospital events. The convenience sample of parent–child dyads included one parent with a CCS off all treatment for at least 1 year (n 11). Non-CCS families were recruited through paper and digital flyers posted in the greater Houston and Austin areas, TX, USA. Non-CCS (n 29) and their parents were also recruited for comparison. All participants met the following eligibility criteria: (i) child was aged 5–17 years; (ii) parent could read and speak English; (iii) parent self-reported preparing meals for the child at least one time per week on average; and (iv) no one in the home had food allergies. The study was reviewed and approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center (PA16-0995).
Procedure
Video observations of cooking events
Each participating dyad scheduled and completed a video observation session during a normal evening meal preparation event. Video sessions were scheduled according to participant availability. Prior to video session scheduling, parents were asked to report their most commonly made dishes. Upon scheduling, parents were asked to select and prepare one of the commonly reported dishes or something similar. Parent informed consent and child assent were completed in the home before filming. Equipment was arranged in a participant’s kitchen and included: (i) a wide-angle camera on a tripod positioned to capture the entire kitchen area; (ii) a wireless, lapel-worn microphone placed on the parent participant; and (iii) a small, chest-worn body camera (Sun eButton) to provide another angle on cooking behaviours(Reference Raber, Patterson and Jia19,Reference Sun, Fernstrom and Jia20) . Parents were instructed to prepare their planned meals and to explain what they were doing and why into the microphone during food preparation. One to two observers were present to take notes and ask for clarification as needed during the session. Prior to beginning preparation tasks, all parents were asked to state what dish they were making and why they chose to make the dish. Parents were also encouraged to talk about their general cooking practices and any factors impacting their cooking habits. Video session recordings were analysed using the Healthy Cooking Index (HCI), based on a previously developed conceptual framework of healthy cooking(Reference Raber, Chandra and Upadhyaya21).
Healthy Cooking Index
The HCI is an index of food preparation practices that registers points for specific behaviours, which are summed to create a composite score of cooking behaviour. The HCI coding system registers a simple +1 for the demonstration of healthy behaviours and −1 for the demonstration of unhealthy behaviours. The construction of the conceptual framework underpinning the HCI has been detailed elsewhere(Reference Raber, Chandra and Upadhyaya21). The items in the HCI are relevant to CCS given their generally poor diet quality and increased risk of CVD, unhealthy weight gain and secondary neoplasms(Reference Aldhafiri, Al-Nasser and Al-Sugair13,Reference Cohen, Wakefield and Fleming17,Reference Co-Reyes, Li and Huh22) . Specific healthy and unhealthy behaviours from the index are detailed in Fig. 1.
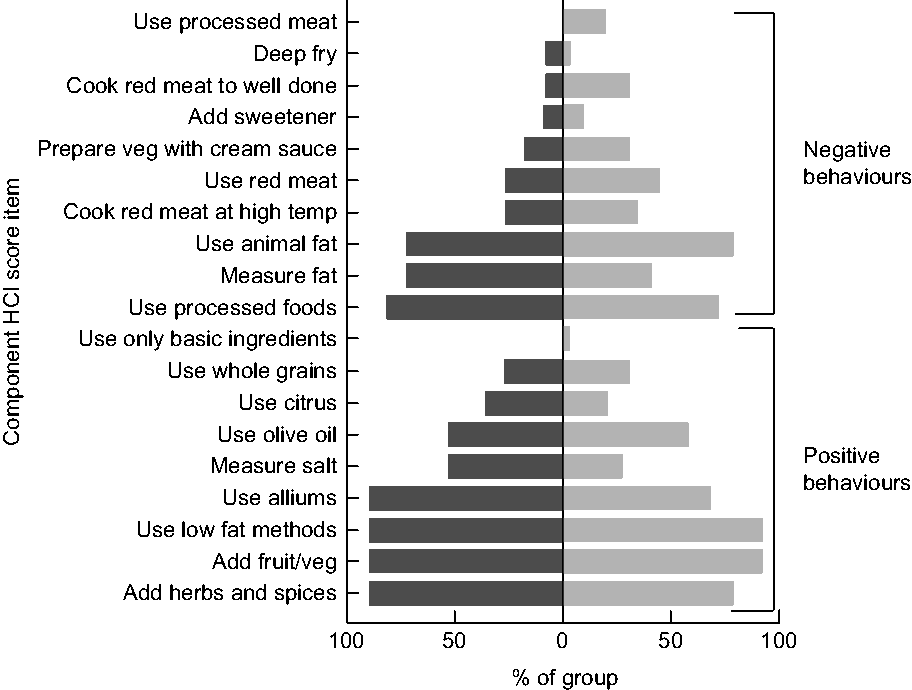
Fig. 1 Healthy cooking practices based on component score items of the Healthy Cooking Index (HCI), by group (![]() , childhood cancer survivors (CCS);
, childhood cancer survivors (CCS); ![]() , non-CCS), among dyads comprising a parent and their 5–17-year-old child (CCS, n 11; non-CCS, n 29), greater Houston and Austin areas, TX, USA, October 2017–June 2018
, non-CCS), among dyads comprising a parent and their 5–17-year-old child (CCS, n 11; non-CCS, n 29), greater Houston and Austin areas, TX, USA, October 2017–June 2018
Study measures
During video sessions, observers estimated the ingredient amounts used in meals and clarified the contents of certain ingredients with participants as necessary. Participants were asked to report the number of servings yielded from each recipe. Nutrient composition of final meals was analysed using the Nutrient Data System for Research software (NDSR 2017; University of Minnesota, Minneapolis, MN, USA). Immediately after completion of cooking, parents completed a demographics and family characteristics questionnaire which included items on parent age, gender, education, ethnicity, income level, marital status, and child age and gender, as well as family meal habits. Time off treatment and diagnosis information were collected from CCS parents. Also, a scale and stadiometer were brought to participants’ homes and anthropometric measurements were collected by trained project staff. Raw BMI was calculated according to the formula weight/height2 and BMI percentiles obtained through comparison with the Centers for Disease Control and Prevention growth charts(Reference Ogden, Kuczmarski and Flegal23).
Data analysis procedures
Demographic and family characteristics, as well as cooking habits, were examined by CCS status. Differences between categorical characteristics of the two groups were examined using χ 2 tests. Nutrient values of meals including carbohydrate, fat, saturated fat, protein, sugar, fibre, energy and energy density were examined. Resulting videos were coded for healthy cooking practices using the HCI. HCI scores in CCS and non-CCS families were compared using one-way ANCOVA controlling for dissimilarities between the two groups. Frequencies of individual behaviours from the HCI score were examined by group. Comparative and descriptive statistics were performed with the statistical software package IBM SPSS Statistics for Windows version 25.0.
Results
Participant demographic and family meal characteristics
Characteristics of participants are shown in Table 1. Differences between the CCS and non-CCS group included child race, with the non-CCS group being more racially diverse (P = 0·041), and number of children in the household, with CCS households more likely to have only one child (CCS = 50·0 %; non-CCS = 10·3 %, P = 0·021). Most families in both groups owned their homes and earned more than $US 60 000 per year (Texas average is $US 59 206)(24). Parents in both groups reported having dinner together as a family on four or more evenings during a typical Monday–Friday (CCS = 72·7 %; non-CCS = 68·9 %). With regard to number of days the parent cooked the child’s evening meal, the majority of both groups reported cooking five or more days (CCS = 63·7 %; non-CCS = 55·2 %).
Table 1 Demographics and family characteristics, by group (childhood cancer survivors (CCS) and non-CCS), among dyads comprising a parent and their 5–17-year-old child (CCS, n 11; non-CCS, n 29), greater Houston and Austin areas, TX, USA, October 2017–June 2018
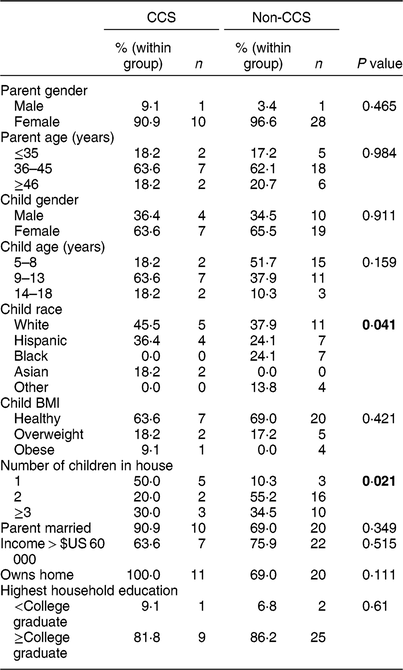
P values represent χ 2 tests examining significant differences between the two groups. Significant P values (P < 0·05) are indicated in bold.
Participant healthy cooking practices
CCS summative HCI scores (possible range = −9 to +10) ranged from −1 to +7 (mean = 3·55, sd = 2·876). Non-CCS scores ranged from −4 to +7 (mean = 1·90, sd = 2·677). No significant difference was detected between the groups, even when controlling for major between-group differences (F = 1·902, P = 0·175). Items from the HCI score coding system were explored by group (Fig. 1). Both groups demonstrated higher use of animal fats (CCS = 72·7 %; non-CCS = 79·3 %) and processed foods (CCS = 81·8 %; non-CCS = 72·4 %), and lower use of whole grains (CCS = 27·3 %; non-CCS = 31·0 %) and basic ingredients (CCS = 0 %; non-CCS = 3·4 %). Overall, CCS and non-CCS participants demonstrated similar healthy cooking practices based on both the summative and component HCI score items.
Nutrient analysis of the meals prepared during the video sessions revealed comparable meal nutrient compositions between the CCS and non-CCS prepared meals. Both samples were comparable to US averages, and all groups demonstrated dinners with more sugar, fat, saturated fat, carbohydrate and protein content per serving than nationally (US) recommended dietary intakes (Table 2).
Table 2 Mean meal nutrient profile (per serving of evening meal), by group (childhood cancer survivors (CCS) and non-CCS), and comparison with national average and nationally recommended dietary intakes, among dyads comprising a parent and their 5–17-year-old child (CCS, n 11; non-CCS, n 29), greater Houston and Austin areas, TX, USA, October 2017–June 2018
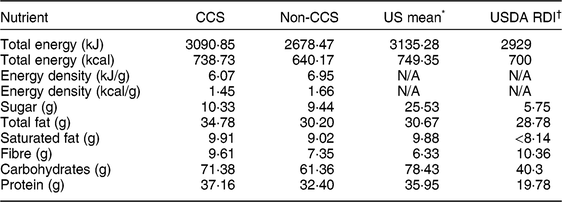
USDA, US Department of Agriculture; RDI, Reference Dietary Intake; N/A, not applicable.
Mean nutrient amounts are reported per serving of dinner. Ingredients and amounts were taken from observer notes.
* Publicly available data from the USDA, Agricultural Research Service (2016) Nutrient Intakes from Food and Beverages: What We Eat in America, NHANES 2013–2014 (https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1314/Table_21_DIN_GEN_13.pdf).
† US Department of Health and Human Services and USDA (2015) 2015–2020 Dietary Guidelines for Americans, 8th ed. (https://health.gov/dietaryguidelines/2015/guidelines/).
Discussion
The present study examined the food preparation habits of eleven CCS and twenty-nine non-CCS parent–child dyads through audio/video observation and questionnaires. In this small sample, CCS families did not show substantial differences from non-CCS families with regard to cooking frequency, family meal frequency, meal nutrition or cooking practices. Our findings offer exploratory data of CCS family cooking practices and elucidate key areas for consideration when developing or adapting practical nutrition interventions for this population.
An important influence shaping a child’s diet is the family food environment and family meals(Reference De Wit, Stok and Smolenski25,Reference Hammons and Fiese26) . In the present study, parent participants reported commonly eating dinner together during the week at home and cooking meals at least five days per week. This suggests home cooking practices and family meals may be an important target for nutrition interventions in the CCS population as home-prepared foods represents a large portion of eating events. Interest in cooking as a nutrition intervention target has increased in the past several decades, although the impact of interventions has varied(Reference Hersch, Perdue and Ambroz27,Reference Reicks, Kocher and Reeder28) . In particular, a large randomized trial focusing on family meal frequency showed limited effects on nutrition and dietary outcomes in children(Reference Fulkerson, Friend and Horning29).
Nutritional interventions for CCS that incorporate cooking components should be carefully designed to maximize acceptability and efficacy in this population. Previous survey studies have suggested CCS are interested in healthy eating programmes, in particular computer-delivered interventions, and interventions with parents(Reference Badr, Chandra and Paxton30,Reference Badr, Paxton and Ater31) . Previous research suggests online cooking videos have the potential to improve eating habits among adults(Reference Adam, Young-Wolff and Konar32). Digitally delivered cooking interventions that engage both parents and CCS may be of particular interest and benefit to CCS. Our group has developed an online cookbook specifically for CCS that may be utilized in future interventions(Reference Li, Raber and Chandra33). The HCI used in the present study provides a guide for developing online cooking education module content, as we have demonstrated these practices are relevant to CCS.
Limitations include the limited sample size and use of a convenience sample. Recruitment of CCS for the study was challenging due to a recent hurricane in the region, discomfort with home observations among survivors and changing contact details as children transitioned to survivorship care after treatment. Participants may differ from the general population given their willingness to have researchers enter their homes and record their behaviours. Our sample was more educated and earned higher incomes that the average family in the area. Age ranges and inclusion criteria were kept broad to maximize recruitment potential for the study. The present study was not powered to identify significant differences between the CCS and non-CCS groups; therefore findings are exploratory. Finally, height and weight were collected from children, but ancillary data on conditions/medications that may influence weight were not collected.
The present study is the first to explore food preparation practices in CCS families. Strengths of this project include the use of objective observational cooking data collected from participant homes, meal nutrient composition data, inclusion of a comparison group, and an evidence-based index to identify key cooking practices relevant to CCS long-term well-being(Reference Raber, Chandra and Upadhyaya21). The study provides rich formative data for the development of future nutrition interventions among CCS.
Acknowledgements
Acknowledgements: The authors would like to thank all participants for taking part in this study. Financial support: This project has been supported by the James and Lois Archer foundation, the Center for Energy Balance in Cancer Prevention and Survivorship, Duncan Family Institute for Cancer Prevention and Risk Assessment, and the National Cancer Institute of the National Institutes of Health (grant number R25CA057730). This work is a publication of the US Department of Agriculture/Agricultural Research Service (USDA/ARS) Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX, and has been funded in part with federal funds from the USDA/ARS under Cooperative Agreement No. 58-3092-5-001. These funding organizations had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: M.R. developed the project and research plan, and conducted recruitment, data collection, study management and manuscript construction. K.C. assisted in data collection and recruitment. T.B., S.V.S, V.S. and C.M. offered guidance and content expertise throughout the development, implementation and analysis of this project; they also supported the drafting and editing of this manuscript. M.R. supported project development and patient recruitment. J.C. oversaw the development and implementation of all aspects of this project, and supervised analysis and manuscript production. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Texas MD Anderson Cancer Center Institutional Review Board (PA16-0995). Written informed consent was obtained from all participants.





