Introduction
Neglected tropical diseases (NTDs) are some of the most common infections of poor people in impoverished communities not only in the poorest low- and middle-income countries (LMICs) but also in the richer G20 countries (Hotez, Reference Hotez2017). Several of these diseases (lymphatic filariasis, onchocerciasis, soil-transmitted helminths, schistosomiasis and trachoma) are amenable to preventive chemotherapy (PC). With progress in the delivery of treatments, there are opportunities to transition from control to elimination of these diseases, or to verify the absence of further transmission as outlined in the World Health Organization (WHO) 2020 NTD roadmap (WHO, 2012). As we approach elimination targets for PC NTDs in many settings, programmes face numerous challenges that can be grouped into core categories – biological and technological, political and social, and environmental (Bockarie et al. Reference Bockarie, Kelly-Hope, Rebollo and Molyneux2013; Webster et al. Reference Webster, Molyneux, Hotez and Fenwick2014). This paper explores how the health system can become a mediator between such challenges at the local level and those at the national/international level. Specifically, we argue how responsive and resilient health systems are essential to reach the aspiration of the NTD ‘endgame’ as articulated in the sustainable development goals (SDGs).
Global shocks: a disrupting factor for NTD control
Since 2007, a series of global events that span the social, environmental and political, present a challenging set of circumstances against which health systems, encompassing NTD programmes, have been required to adapt such as the global financial crisis, increased conflict, drought and the emergence of terror organizations. These events have induced almost unprecedented migration, creating refugee and displaced persons crises and stressing already weak and fragile health systems. The impact of such crises was exemplified during the 2014 Ebola (EVD) epidemic in Liberia, Sierra Leone and Guinea. EVD originated in remote and difficult to access communities, with complex health-seeking pathways, a weak and poorly resourced health system, and limited surveillance capacity. In these contexts, the health system faced an unprecedented challenge given the high case mortality rates and wide-reaching psycho-social impacts. Diversion of resources to control this EVD epidemic caused a necessary acute polarization of global and national health systems and focussed attention away from other chronic problems, such as NTDs. This presented an impediment to NTD control in the affected regions, specifically in Sierra Leone where significant progress had previously been made (Hodges et al. Reference Hodges, Koroma, Sonnie, Kennedy, Cotter and Macarthur2011; Pose and Rabinowitch, Reference Pose and Rabinowitch2014), as well as in Guinea and Liberia where successes in onchocerciasis control has led to the emergence of NTD programmes supported initially by Non-Governmental Development Organizations (NGDOs) (Bogus et al. Reference Bogus, Gankpala, Fischer, Krentel, Weil, Fischer, Kollie and Bolay2016; Thomas et al. Reference Thomas, Kollie, Koudou and Mackenzie2017). Thus, global and national shocks frequently become a significant disrupting factor for NTD control, and create a challenge for the ability of health systems to respond appropriately to changing population needs, whilst also supporting the ‘everyday resilience’ that allows for the ongoing functioning of routine activity, such as immunization and NTD programme delivery (Gilson et al. Reference Gilson, Barasa, Nxumalo, Cleary, Goudge, Molyneux, Tsofa and Lehmann2017).
Changing global dynamics: a need for better control of vectors
Biological shifts that allow for the rapid emergence and potential spread of vector-borne viruses, are exemplars of another type of challenge facing the global health community. The emergence of Zika virus in the Americas in 2016, has emphasized the need for better surveillance systems and improved awareness of the risks of vector-borne viruses. The expansion of the Aedes populations also poses significant threats to the control of dengue and Chikungunya, as these Aedes transmitted viruses are expanding their range, for example, into the Gulf Coast States of the USA. Yellow fever epidemics have also occurred more recently in central Africa and Brazil and as a result, the WHO have initiated an Eliminating Yellow Fever Epidemic (EYE) strategy with the aim to eliminate such epidemics (WHO, 2017a). Despite such changing biological dynamics, there have been many historical successes in vector control dating back to 1904 including the control of malaria and yellow fever in Panama; malaria in Brazil (with the “eradication” of Anopheles gambiae); onchocerciasis in West Africa; control of dengue in Singapore and Cuba; some initial impact of vector control on Triatome transmission of Chagas’ Disease in the Southern Cone of the Americas; filariasis control in the Solomon Islands because of indoor residual spraying (IRS) against malaria vectors (Webber, Reference Webber1979); and success in filariasis programmes through vector control using bed-nets in Nigeria, The Gambia and Zambia (Blackburn et al. Reference Blackburn, Eigege and Gotau2006; Eigege et al. Reference Eigege, Kal, Miri, Sallau, Umaru, Mafuyai, Chuwang, Danjuma, Danboyi, Adelamo, Mancha, Okoeguale, Patterson, Rakers and Richards2013; Rebollo et al. Reference Rebollo, Sambou, Thomas, Biritwum, Jaye, Kelly-Hope, Escalada, Molyneux and Bockarie2015; Nsakashalo-Senkwe et al. Reference Nsakashalo-Senkwe, Mwase, Chizema-Kawesha, Mukonka, Songolo, Masaninga, Rebollo, Thomas, Bockarie, Betts, Stothard and Kelly-Hope2017). Perhaps the most notable impact of vector control, however, has been the reduction in malaria morbidity and mortality attributed to long-lasting insecticide-impregnated bed-nets (LLIN) in sub-Saharan Africa. An estimated 68% of the decline in malaria prevalence was attributed to vector control (including IRS) between 2000 and 2015 (Bhatt et al. Reference Bhatt, Weiss and Cameron2015; WHO, 2016).
Despite these historic successes, however, and the clear impact that vector control can have in addressing many global health challenges, vector control has been side-lined in attempts to control and eliminate many NTDs. Many elimination targets focus on a PC strategy with limited recognition that infections are acquired by insect bites or contact with freshwater snail habitats. In elimination programmes, transmission control is the key and too often the opportunity to address transmission has been underrecognized. The concept of mass drug administration (MDA) in lymphatic filariasis and onchocerciasis is designed to reduce the circulating populations of microfilariae to reduce transmission without vector control; however, the inclusion of vector control initiatives would greatly enhance the progression towards the elimination targets (Hollingsworth et al. Reference Hollingsworth, Adams and Anderson2015). Progress in control of vectors of other parasitic infections, such as human African trypanosomiasis, i.e. Glossina spp. (Tirados et al. Reference Tirados, Esterhuizen, Kovacic, Mangwiro, Vale, Hastings, Solano, Lehane and Torr2015), Chagas’ Disease (Dias et al. Reference Dias, Silveira and Schofield2002) and visceral leishmaniasis, phlebotomine sandflies (Coleman et al. Reference Coleman, Foster, Deb, Pratap Singh, Smail, Shivam, Ghosh, Dunkley, Kumar, Coleman, Hemingway, Paine and Das2015), are critical to the achievement of elimination targets.
While there is no doubt that vector control should be a key pillar of NTD interventions, the challenges of emerging insecticide resistance in Anopheles populations in Africa, pose a threat to the continued impact of bed nets and LLINs (Hemingway et al. Reference Hemingway, Ranson, Magill, Kolaczinski, Fornadel, Gimnig, Coetzee, Simard, Roch, Hinzoumbe, Pickett, Schellenberg, Gething, Hoppé and Hamon2016). Furthermore, there has been limited recognition of the role of vectors and the need to increase the focus on transmission control. For example, whilst the WHO roadmap emphasizes the need for new drugs and diagnostics, as well as revitalized tools for monitoring and evaluation, demand for additional vector control tools and approaches has been noticeably absent (WHO, 2012). However, in its third NTD report WHO did highlight the additional cost of vector control which would be required to meet some of the control targets for infections such as dengue (this report was issued prior to the emergence and spread of Zika virus). More recently, WHO has responded to the increased threat of vector-borne infections and published a draft Global Vector Control Response 2017–2030 (WHO, 2017b). This document highlights the contributions which vector control has historically made to public health and links vector control interventions to the SDGs.
Environmental shifts
Rapid environmental change resulting in a less stable and unpredictable climate has also impacted on NTD epidemiology. These changes include: deforestation, mineral extraction and exploitation; habitat destruction and desertification; changes in water resource availability; severe climate change driving major flood events and landslides (e.g. China, Bangladesh, Sierra Leone); more frequent droughts (e.g. Africa) with consequences for food security; and associated threats to livestock husbandry and animal well-being as traditional agricultural practices create local conflicts over land and water resources. Environmental shifts coupled with changing global patterns and human–animal interactions reinforce the need for a ‘one health’ approach. For example, the World Health Assembly (WHA) resolution of 2013 on NTDs emphasized that the control of zoonotic diseases as a core NTD strategy whilst the research priorities were identified to address the major zoonotic diseases – rabies, echinococcosis, taeniasis and neurocysticercosis in the WHO NTD portfolio (WHO, 2015a).
The neglected within the neglected: innovative approaches required for impact
Thinking beyond preventive chemotherapy diseases
To date, NTD efforts have focused on the up-scaling of PC by MDA, fuelled by large-scale drug donations. This has posed challenges for the management of other NTDs, which require intensive disease management (IDM) such as the trypanosomiases, Buruli ulcer, leprosy and the leishmaniases which have not been attributed the same level of resources despite their capacity to be fatal if untreated and the donation of curative drugs. Such IDM diseases can be more focal in their geographical distributions and thus present a need for more nuanced and directive interventions and a need for greater clinical expertise for diagnosis and treatment. There are, however, serious deficits in the therapeutic armamentarium for IDM diseases, which have led to continued investment in and advocacy for, this critical cause by the Drugs for Neglected Diseases initiative (DNDi) (www.dndi.org) and significant progress has been made in reducing the burden of human African trypanosomiasis and visceral leishmaniasis (WHO, 2016; Molyneux et al. Reference Molyneux, Savioli and Engels2017). However, those who remain with chronic clinical, and often irreversible symptoms because of such diseases, urgently require follow-up action and support. Furthermore, the foundation of the NTD programme through ‘vertically’ implemented MDA (which frequently by-passes health systems infrastructure) further compounds this challenge, as staff working within routine health services often lack clinical knowledge and capacities to be able to diagnose and manage such conditions. In these situations, a health systems as usual response is insufficient to address complex chronic conditions and innovative approaches are needed to reach an often diverse sub-set of a population who are disproportionately affected by significantly disabling and potentially life-threatening NTDs.
A need for alternative strategies to enhance preventive chemotherapy mass drug administration
In West and Central Africa, occurrence of lymphatic filariasis and onchocerciasis in areas of Loa loa endemicity, pose a significant challenge to elimination goals because of the severe adverse events (SAEs) associated with the impact of ivermectin on individuals with high parasitaemias of Loa microfilaria (>30 000 ml−1), exacerbating risk of encephalopathy (Gardon et al. Reference Gardon, Gardon-Wendel, Demanga-Ngangue, Kamgno, Chippaux and Boussinesq1997; Boussinesq, Reference Boussinesq2006). Over nearly two decades, extensive studies have concentrated research approaches on seeking to understand the pathology of SAEs as well as searching for strategies to identify individuals at risk, and where lymphatic filariasis is co-endemic, to define alternative strategies. Such strategies have included the use of twice-a-year albendazole, supplemented with vector control (Pion et al. Reference Pion, Chesnais, Weil, Fischer, Missamou and Boussinesq2017). There has, however, been limited consideration of alternative approaches by investing in testing the potential of reducing transmission by the vector Chrysops; if the transmission of L. loa in areas of high Loa endemicity could be reduced, the numbers of individuals with high to moderate parasitaemias would decline, hence the risk of SAEs would be reduced as high adult worm loads (driven by high levels of transmission) would also reduce. The neglect of the Chrysops dimension in considering the ‘Loa problem’ is reflected in the fact that the first review of Chrysops biology for over 50 years was published in 2017 (Kelly-Hope et al. Reference Kelly-Hope, Paulo, Thomas, Brito, Unnasch and Molyneux2017). This recent review includes suggestions for implementing vector control, e.g. the use of ‘tiny targets’, which are widely deployed for Glossina control (Tirados et al. Reference Tirados, Esterhuizen, Kovacic, Mangwiro, Vale, Hastings, Solano, Lehane and Torr2015) and could have significant potential for reducing Chrysops populations. Thus, alternative strategies that prioritize vector control, as well as new drug distribution mechanisms, are likely to be essential in addressing more complex NTD scenarios.
Thinking beyond the parasite: addressing long-term manifestations of NTDs
There has been considerable debate about the burden attributed to NTDs by the global burden of disease (GBD) studies (Murray et al. Reference Murray, Vos, Lozano, Naghavi, Flaxman, Michaud, Ezzati, Shibuya, Salomon, Abdalla, Aboyans, Abraham, Ackerman, Aggarwal, Ahn, Ali, Alvarado, Anderson, Anderson, Andrews, Atkinson, Baddour, Bahalim, Barker-Collo, Barrero, Bartels, Basáñez, Baxter, Bell and Benjamin2012) and the discrepancy between earlier estimates of burden (Hotez et al. Reference Hotez, Alvarado, Basáñez, Bolliger, Bourne, Boussinesq, Brooker, Brown, Buckle, Budke, Carabin, Coffeng, Fèvre, Fürst, Halasa, Jasrasaria, Johns, Keiser, King, Lozano, Murdoch, O'Hanlon, Pion, Pullan, Ramaiah, Roberts, Shepard, Smith, Stolk, Undurraga, Utzinger, Wang, Murray and Naghavi2014). The estimates by the GBD 2010 attributed some 27 million disability-adjusted life years (DALYs) to NTDS whilst a study in 2014 (which included the 17 WHO NTDs as well as other NTD conditions) attributed 47.9 million DALYs (Hotez et al. Reference Hotez, Alvarado, Basáñez, Bolliger, Bourne, Boussinesq, Brooker, Brown, Buckle, Budke, Carabin, Coffeng, Fèvre, Fürst, Halasa, Jasrasaria, Johns, Keiser, King, Lozano, Murdoch, O'Hanlon, Pion, Pullan, Ramaiah, Roberts, Shepard, Smith, Stolk, Undurraga, Utzinger, Wang, Murray and Naghavi2014). Calculations of the mortality associated with NTDs according to the GBD gives an annual mortality of 150 000, a figure which has been challenged (Molyneux et al. Reference Molyneux, Savioli and Engels2017) as deaths from trematode-induced cancers, epilepsy (caused by neurocysticercosis) rabies, and snake bite were not included. The WHO figure of schistosomiasis associated deaths in Africa has also been estimated to be 20 times higher than GBD estimates, as none of the conditions have been correctly attributed to the NTD group of diseases but included as injuries, cancers and neurological conditions.
These discrepancies could reflect the impact of interventions over the past decade but the attribution of the disability weights and overall mortality of NTDs remains controversial, as disability weights and prevalence are key drivers in the calculations of DALYs. Gross underestimates of the prevalence of cutaneous leishmaniasis (Bailey et al. Reference Bailey, Mondragon-Shem and Hotez2017) exemplifies this problem where residual scarring of a cured condition is not included, despite the social and mental health sequelae caused by the condition (Bailey et al. Reference Bailey, Mondragon-Shem and Hotez2017). The mental health co-morbidities have recently been identified as being of increased significance as a major and chronic morbidity (Litt et al. Reference Litt, Baker and Molyneux2012) in many of the NTDs. The mental health burden estimated for lymphatic filariasis is two to three times higher than those estimated by the GBD study for this condition using highly conservative figures. In addition, the mental health burden which caregivers suffer in the care of chronic NTD patients has only been assessed in filariasis (Ton et al. Reference Ton, Mackenzie and Molyneux2015). In the study of DALYs averted for ten major NTDs (de Vlas et al. Reference De Vlas, Stolk, le Rutte, Hontelez, Bakker, Blok, Cai, Houweling, Kulik, Lenk, Luyendijk, Matthijsse, Redekop, Wagenaar, Jacobson, Nagelkerke and Richardus2016), impaired cognitive development due to soil-transmitted helminths and schistosomiasis infection, mental health morbidity, discrimination and stigma due to disfigurement, social exclusion resulting in poor marital prospects, the impact of catastrophic health expenditures and economic impact of reduced ability to work, were not included in the DALYs averted calculations exemplifying the need to adopt a more holistic appreciation of the overall NTD burden (Litt et al. Reference Litt, Baker and Molyneux2012). Conversely, the apparent absence of consideration of NTDs by the mental health community as a cause of and a contribution to global mental health morbidity, and the need to address the patient and caregiver needs to give the existence of so many chronic NTD patients, should bring NTD and mental health communities together (Ferrari et al. Reference Ferrari, Charlson, Norman, Patten, Freedman, Murray, Vos and Whiteford2013). Mental health is already projected to be the largest cause of Global Disease Burden by 2030, without consideration of the contribution of NTDs. Including NTDs within these mental health projections are important and will further elevate the serious comparative lack of recognition for both sets of diseases.
Furthermore, where completed, measures of disease associated morbidity (including mental health and disability) are frequently quantitatively defined. Given the complexity and multifaceted manifestations of NTDs at both the individual and community level, research that allows for more nuanced understandings from the perspectives of affected individuals is important (Reidpath et al. Reference Reidpath, Allotey and Pokhrel2011). Historically, the limited acknowledgement of the lifelong morbidity associated with such diseases (Mieras et al. Reference Mieras, Anand, van Brakel, Hamilton, Martin Kollmann, Mackenzie, Mason and Wickenden2016), has led to the highly medicalized focus of morbidity measurement and programme implementation, e.g. the need to restore sight or reduce physical impairment related to NTDs such as lymphatic filariasis. Currently, the shift within international NTD policy and programming to recognize the need for a holistic approach to the control and management of NTDs through the presentation of strategies for disease management, disability and inclusion (DMDI) (Mieras et al. Reference Mieras, Anand, van Brakel, Hamilton, Martin Kollmann, Mackenzie, Mason and Wickenden2016) represents the opportunity to develop morbidity measures that are responsive to the needs and experiences of affected populations, and how they vary by differing axes of inequality, such as age, gender, stage of disease, experience of disability and whether living with one or several NTDs. Only when the complexities of individual and household realities are understood and measured in relation to NTDs are the needs of affected populations likely to be prioritized and addressed.
Harnessing partnerships and political commitments
The importance of partnerships
There are opportunities for holistic multi-sectoral action to support robust, resilient and responsive health systems responses to mediate challenges and sustain NTD control. Notably, NTD partnerships require financial resources and management time and the overall financial envelope has not increased; in 2010, the proportion of Official Development Assistance (ODA) committed to NTD programmes was 0.6% (Liese and Schubert, Reference Liese and Schubert2009). Funds over the last 7 years have increased despite the higher profile for NTDs (Liese et al. Reference Liese, Houghton and Teplitskaya2014). However, the diversity of interests and the numbers of partners involved has expanded significantly and this reflects the increasing recognition of the importance of these diseases. In addition, there is an ever more complex partner and donor landscape both in terms of implementation of disease-specific approaches and integrated country programmes. A recent estimate provided by the infontd website (www.infontd.org) has identified over 2609 projects on NTDs in 113 countries involving 73 different partners.
Disease-specific partnerships have evolved and expanded in the last decade to provide focal points for endemic country partners, ministries of health, researchers and donors including WHO and the NGDO community (Liese et al. Reference Liese, Rosenberg and Schratz2010). Such partnerships have an important advocacy role and enhance communication, networking, and technological advancements. The expansion of NGDO interest in NTDs has seen the development of the NNN-NGDO/NTD/Network over recent years (www.ntd-ngonetwork.org), as well as the Coalition for Operational Research COR-NTD (www.ntdsupport.org/cor-ntd) which identifies and supports operational research priorities. Research partnerships that span all areas of the translational research spectrum are also of critical importance in increasing the adaptability of NTD programmes in relation to emergent challenges.
Partnerships, however, must be based on parity of esteem of partners, transparency of management, the capacity to recognize sensitivities and manage them, regular communication and adequate resourcing. Most importantly, they should prioritize equity between colleagues in the global north and global south and be responsive to the needs and priorities of health systems and populations in LMICs who face the largest burden of NTDs. The significance of cross-cutting issues in the control of NTDs such as links to the WASH sector (WHO, 2015b), education, environment, agriculture and livestock, are important prerequisites to meeting the United Nations SDGs (Bangert et al. Reference Bangert, Molyneux, H Lindsay, Fitzpatrick and Engels2017). Partnerships that include expertise across and within such sectors and prioritize multi-disciplinary and multi-sectoral action are of critical importance if we are to reach the NTD ‘endgame’. Below we illustrate the range of strategic partnerships needed across the translational research continuum: T1 (basic research), T2 (human/clinical research), T3 (evidence into practice) and T4 (practice to policy).
Partnerships for drug development (T1–T2)
The anti-Wolbachia (A·WOL) consortium reflects the importance of building a partnership for drug development using the comparative advantage of academic institutions and industry partners. The partnership focuses on the development of an effective macrofilaricide against adult filaria worms based on the efficacy of antibiotics against Wolbachia endosymbionts of Onchocerca and Wuchereria (www.awol.lstmed.ac.uk). Having demonstrated the efficacy of doxycycline as a macrofilaricide, A·WOL have promising candidate alternatives which can shorten the duration of treatment needed to secure adult worm death or permanent sterilization of infection. Such alternatives include high-dose rifampicin (Aljayyoussi et al. Reference Aljayyoussi, Tyrer, Ford, Sjoberg, Pionnier, Waterhouse, Davies, Gamble, Metugene, Cook, Steven, Sharma, Guimaraes, Clare, Cassidy, Johnston, Myhill, Hayward, Wanji, Turner, Taylor and Ward2017) and a new entity TylAMac™ (Turner et al. Reference Turner, Ford, Tyrer, Sjoberg, Gamble, Cook, von Geldern, Marsh, Wanji, Ward and Taylor2015).
Partnership for innovation and co-implementation across the disease programmes (T1–T4)
The Integrated Vector Control Consortium (IVCC) (www.ivcc.com) is an example of the development of novel new vector control products which will ensure that vector control successes can be maintained in the future. IVCC is the only partnership involving academia, WHO, and the pesticide industry, focused on seeking alternative products for vector control which contrast to the several partnerships formed for addressing new drugs and diagnostics such as DNDi (www.dndi.org), the Medicine for Malaria Venture (MMV) (www.mmv.org), and the Foundation for Innovative New Diagnostics (FIND) (www.finddx.org). An irony upon which it is worth reflecting, is that a single new chemical entity effective against vectors, as synthetic pyrethroids have been over several decades, will impact on all vectors of infectious agents. Hence, it could be argued that proportionately more resources should be devoted to the search for new vector control products given the extent of pyrethroid resistance (Hemingway et al. Reference Hemingway, Ranson, Magill, Kolaczinski, Fornadel, Gimnig, Coetzee, Simard, Roch, Hinzoumbe, Pickett, Schellenberg, Gething, Hoppé and Hamon2016). Targeting vectors will have the most effective impact on transmission control and contribute proportionately more to elimination. Hence a ‘pan’ vector control product would have a potentially greater impact than a drug for one specific condition.
Building on the successes of vector control, the operationalization of the strategic partnership between the lymphatic filariasis and the malaria programme in Nigeria has shown the benefit of co-implementation through the combined distribution of LLIN. The key success of this programme was the endorsement in the policy of a synergistic approach of distribution of MDA and LLIN (Dean et al. Reference Dean, Page, Hawkins, Stothard, Thomson, Wanji, Gyapong, Anagbogu, Molyneux and Theobald2016).
Partnerships for Health Systems Strengthening (T3–T4)
COUNTDOWN is a multi-disciplinary health systems implementation research programme which brings together NTD programmes and research institutes to address country priorities and challenges for strengthening NTD programme implementation. COUNTDOWN research takes place in Ghana, Cameroon, Nigeria and Liberia. For example, in Nigeria, following a situational analysis, key challenges were identified in four broad areas including: (i) co-ordination and collaboration; (ii) financial and non-financial resource mobilization; (iii) long-term multi-context community engagement; and (iv) human resources management and motivation (www.countdownonntds.org). Following identification of these challenges, COUNTDOWN worked with the Federal Ministry of Health to develop a holistic implementation research package and will work with the Federal and State (Ogun and Kaduna) Ministries of Health to implement a participatory action research cycle to generate innovative approaches for community engagement with the NTD programme, to ensure a detailed understanding of resources required for sustainable programme delivery. This will include a review of the direct and opportunity costs in relation to NTD treatment seeking and provision at the individual and household levels, as well as amongst programme implementers including community directed distributors (CDDs) and frontline health implementers.
Measures of success in improving health
There has been progress in establishing NTDs as a justifiable cause for investment, initiating action-oriented partnerships and establishing a respected ‘brand’ (Molyneux, Reference Molyneux2012) with which policy makers, drug donors and philanthropists were prepared to align. This has provided an enabling environment to progress towards the targets established in the WHO Roadmap (WHO, 2012) and drive towards more equitable health interventions. Efforts have included scientific progress, sustained and high-level advocacy, commitment by the philanthropic community and NGDOs to the cause, which embraced not only health but also poverty alleviation as a development target (Hotez et al. Reference Hotez, Fenwick, Savioli and Molyneux2009; Molyneux et al. Reference Molyneux, Savioli and Engels2017; Bangert et al. Reference Bangert, Molyneux, H Lindsay, Fitzpatrick and Engels2017). This has been enhanced by a consistent message around the role NTDs play in exacerbating poverty and the value for money provided by the interventions, some of which are dependent on donated high-quality assured products with delivery mechanisms able to reach those beyond ‘the end of the road’, often through sustained community commitment. The endorsement by the UN system and its member states following the inclusion of NTDs within the overall health targets of the SGDs has emphasized their importance as impediments to development. This has been highlighted by a detailed analysis of the impact that NTDs have when the other SDG targets are considered – improved access to water and sanitation, the overarching goal of poverty alleviation, improved food security and hunger alleviation, women's empowerment, improved education, absence of discrimination in disability and strengthened partnerships – all interplay with the broad NTD agenda, thereby contributing to the overarching goal of poverty alleviation (Hotez et al. Reference Hotez, Fenwick, Savioli and Molyneux2009; Bangert et al. Reference Bangert, Molyneux, H Lindsay, Fitzpatrick and Engels2017).
The NTD community has also embraced a series of targets for elimination and control as well as a commitment to the eradication of Guinea Worm (dracunculiasis) and yaws following the publication of the WHO Roadmap in 2012. In 2013, the World Health Assembly (2013) endorsed the first comprehensive NTD Resolution on NTDs which outlined the five key strategies required for progress (WHA 66.12) including defining the milestones, linking NTDs to universal health coverage (UHC) and estimating the costs of NTD programmes through a detailed investment case (WHO, 2015c). The progress towards these targets is monitored annually for 10 NTDs through the role of Uniting to Combat NTDs (www.unitingtocombatntds.org). Details of country progress are regularly updated by WHO through reports in the WHO Weekly Epidemiological Record including country by country progress on annual treatments (WHO). NTDs are markers of poverty in many settings, including in poor areas and communities of G20 countries which together with Nigeria, have the highest number of people afflicted by NTDs (Hotez, Reference Hotez2017). Hence progress in their control, elimination or eradication will be a ‘litmus test’ of progress in reducing poverty. NTDs as ‘tracers of equity’ can provide an objective measure of overall progress to SDG goals (Engels, Reference Engels2016) and thus implementing NTD strategies is an essential element of UHC to ‘leave no one behind’.
Making NTDs part of the UHC framing and a key indicator for SDGs is important; but success means turning rhetoric into action. Such realization is likely to involve the use of equity frameworks in programme planning and evaluation and participatory approaches to programme design and appraisal. These approaches require disaggregation of data by key equity markers such as gender, age, poverty and (dis)ability at all levels of the health system and critically using this data to ensure more responsive adaptation of planning. Whilst numbers are useful for identifying bottlenecks to equity, there is also a need to understand the lived experiences and challenges that affected individuals, their families, and frontline health providers (such as community-based drug distributors) face. An example where progress is being made is through the development of WHO's Gender Equity and Rights (GER) toolkit. This toolkit focuses on embedding GER analysis within routine monitoring and evaluation activities conducted by an NTD programme. The process uses routinely collected numerical data around NTD treatment/service provision combined with other equity indicators such as literacy rates or gender parity indicators to identify locations, where programme inequities may exist. More detailed, numerical analysis of indicators is then completed in these areas, before being complemented with a qualitative exploration of implementer (NTD programme staff, community drug distributors, frontline health facility staff) and community experience and perceptions of the NTD programme. Cumulative analysis of numerical data and data that captures the voices of affected populations, is then utilized to develop alternative steps in programme implementation that will increase programme equity in these areas thus creating an ongoing participatory cycle.
Harnessing new technologies
The opportunities to advance the NTD cause through the application of new technologies has been demonstrated by projects which have embraced the rapid expansion of cell phone networks and the reduced costs and availability of smartphones. This is perhaps best exemplified by the Global Trachoma Mapping Project (GTMP) which has enabled most rapid up-to-date information regarding the prevalence and distribution of trachoma globally. Late 2012 to early 2016 saw surveyors collect and transmit data from 2.6 million people in 29 countries using Android smartphones. The GTMP recorded on the International Trachoma Initiative database more districts in 3 years than had been recorded in the previous 12 years, and mapped areas where no data previously existed because of remoteness, insecurity, insufficient funding or competing public health priorities (www.sightsavers.org/gtmp/).
A new approach to detecting patients requiring care for lymphatic filariasis has been developed using the mHealth tool ‘MeasureSMS-Morbidity’ which allows health workers to use cell phones to record and transmit clinical information using short message services (SMS) (Mableson et al. Reference Mableson, Martindale, Stanton, Mackenzie and Kelly-Hope2017) for door-to-door surveys to obtain data on lymphatic filariasis patients in real-time (location, sex, age, clinical condition) in Dar-es-Salaam, Tanzania. This enabled identification, recording and mapping of lymphatic filariasis patients increases the efficiency of planning appropriate morbidity management and disability prevention (MMDP) activities (Mwingira et al. Reference Mwingira, Chikawe, Mandara, Mableson, Uisso, Mremi, Malishee, Malecela, Mackenzie, Kelly-Hope and Stanton2017). Similarly, the use of global positioning system data loggers is likely to become increasingly important to understand the movement of individuals and the dynamics of communities and individual behaviours and consequent transmission ecology (Brant et al. Reference Brant, Okorie, Ogunmola, Ojeyode, Fatunade, Davies, Saka, Stanton, Molyneux, Russell Stothard and Kelly-Hope2018).
Over the last decade the use of remote sensing and satellite imagery for defining habitat and broader ecological associations (Kelly-Hope et al. Reference Kelly-Hope, Bockarie and Molyneux2012; Thomson et al. Reference Thomson, Obsomer, Dunne, Connor and Molyneux2002; Brito et al. Reference Brito, Paulo, Van-Dunem, Martins, Unnasch, Novak, Jacob, Stanton, Molyneux and Kelly-Hope2017) projecting areas of transmission risk, and the need for a micro-mapping overlap stratification approach in Loa endemic areas, has enabled a better understanding of the interactions of ecology and epidemiology. Given the widespread availability of datasets and images of potential importance to the changing disease epidemiology and infection dynamics (forest and land cover, rainfall, altitude, geology), high-resolution imaging methodology is likely to be a more cost-effective approach to mapping and planning than ground truthing, given the rapidly changing ecology which drives the dynamics of transmission of vector-borne infection.
The application of network theory has also been used to ensure that there is a better understanding of the social behaviour of communities to enhance the efficiency of MDA (Chami et al. Reference Chami, Molyneux, Kontoleon and Dunne2013, Reference Chami, Kontoleon, Bulte, Fenwick, Kabatereine, Tukahebwa and Dunne2016, Reference Chami, Ahnert, Kabatereine and Tukahebwa2017). These approaches will enhance the efficiency of delivery of MDA and increase and sustain higher levels of adherence. A further potential approach that could be used for mapping, drug delivery and surveillance is the use of unmanned aerial vehicles (UAVs) or ‘drones’ (Fornace et al. Reference Fornace, Drakeley, William, Espino and Cox2014). UAVs can collect spatial data and their use in ecological research suggests they have potential in infectious disease epidemiology and public health research to provide spatial and temporal data in real-time, enabling an improved understanding of the interactions between disease transmission, vector ecology and environment.
Capacity strengthening
Significant progress on NTD capacity strengthening has been made. With the initial focus to support countries with the largest burdens of NTDs, a strategy which has ensured some progress towards increasing treatments in high burden countries (Nigeria, Ethiopia, Democratic Republic of the Congo, Tanzania and Indonesia). Learning materials have been developed and training courses for national programme managers and district-level teams have been delivered. At Regional WHO levels, significant progress has been achieved though region specific or topic-specific meetings to advocate for a national commitment to NTD capacity development. Building capacity in research as well as in programme implementation has also been essential, whilst the African Research Network for NTDS (www.arntd.org) is a partnership model for research and a critical step forward in meeting this need. We summarize the needs for capacity strengthening in Table 1.
Table 1. The challenges of capacity strengthening

Conclusion
This paper has highlighted the challenges faced by the NTD community in achieving established targets in an ever-changing global world. Challenges that cut across the themes presented in this paper can be grouped into the biological and technological (e.g. the demand for new drugs, diagnostics and alternative vector control); political (e.g. ensuring prioritization of new and old challenges) and social and environmental (e.g. responding to global shocks and thinking beyond the parasite) (Bockarie et al. Reference Bockarie, Kelly-Hope, Rebollo and Molyneux2013; Webster et al. Reference Webster, Molyneux, Hotez and Fenwick2014) as shown in Fig. 1, spanning both the global and local levels.
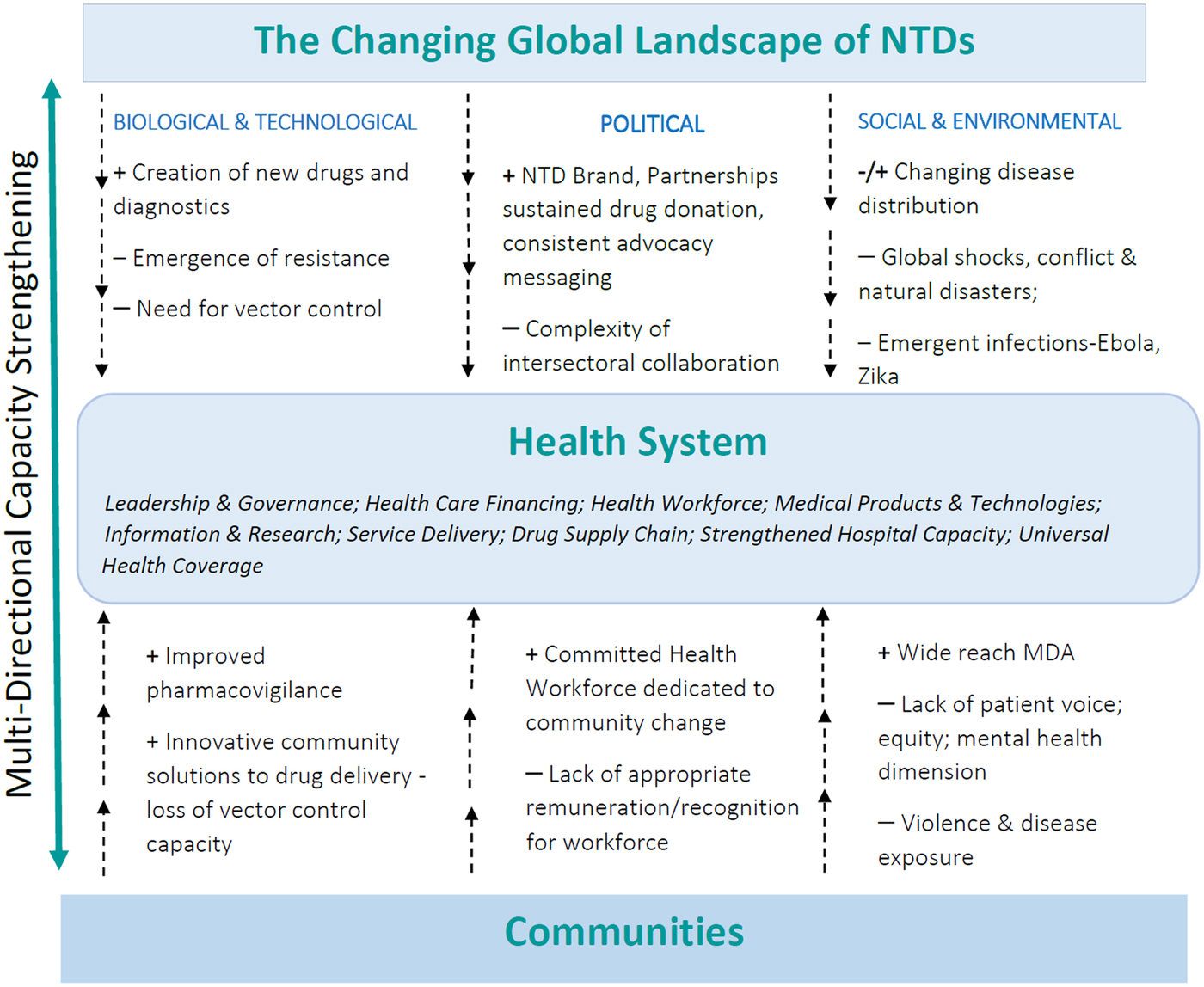
Fig. 1. The Health System as a mediator between the changing global landscape and the community: Moving towards responsive and resilient health systems to reach the NTD ‘end game’.
Health systems ultimately become the mediator between the changing NTD global landscape and the realities of providing health care to poor communities. Multi-directional capacity strengthening, therefore, becomes critical in ensuring progress. As argued, this involves strategic partnership at different stages of the translational research continuum, a prioritization of equity facilitated through ongoing political commitments through the SDGs, and harnessing new technologies. Ultimately equitable partnerships for progress facilitated through multi-directional capacity strengthening are required to both build responsive and resilient health systems and to reach the NTD ‘endgame’.
Financial support
The study was supported in part from the COUNTDOWN programme (grant ID PO 6407), a multi-disciplinary research consortium dedicated to investigating cost-effective, scaled-up and sustainable solutions, necessary to control and eliminate the seven most common NTDs by 2020. COUNTDOWN was formed in 2014 and is funded by UKAID part of the Department for International Development (DFID). The funders played no role in the decision to publish this article.
Conflict of Interest
DHM acknowledges support from GlaxoSmithKline. Other authors declare they have no Conflict of Interest.





