It is well known that dietary iodine intake is required to produce thyroid hormones, but iodine-deficiency disorders still represent a global threat to individuals and societies( 1 ). Since 1922, when Switzerland was the first country to establish a national iodine fortification programme, considerable progress worldwide has been achieved; the number of iodine-sufficient countries has increased to 111 and only thirty countries remain mildly or moderately iodine-deficient( Reference Pearce, Andersson and Zimmermann 2 ). Despite this overall progress, recent data suggest that most pregnant women appear not to be protected against iodine deficiency, even in areas with adequate iodine intake( Reference Gowachirapant, Winichagoon and Wyss 3 , Reference Pessah-Pollack, Eschler and Pozharny 4 ). Therefore, the development of effective prevention and monitoring programmes for iodine-deficiency disorders would be required in all countries because pregnant women represent the most susceptible and vulnerable group and because mild iodine deficiency may impair neuropsychological and motor development in children, preventing them from reaching their full intellectual potential( Reference Bath, Steer and Golding 5 , Reference Pop, Kuijpens and Van Baar 6 ).
A recent meta-analysis has shown that at 5 years of age, children’s intelligence quotient is 7·4 points lower due to iodine deficiency during pregnancy( Reference Bougma, Aboud and Harding 7 ). Econometric models have established that this result greatly impacts not only individuals but also society as a whole, because a 1-point decrease in intelligence quotient has been associated with a persistent 0·11 % annual decrease in per capita gross domestic product, which is connected with a recession in the economy and tends to translate into decreased productivity( Reference Jones and Schneider 8 ). These substantial consequences result from the fact that even mild iodine deficiency disrupts the metabolism of thyroid hormones, which are a critical endocrine regulator of early brain development( Reference Bernal and Nunez 9 ). Thyroid hormones act specifically by regulating the genes that underlie major neurodevelopmental events, including neurogenesis, axon and dendrite formation, neuronal migration, synaptogenesis and myelination( Reference Ausó, Lavado-Autric and Cuevas 10 – Reference Morreale de Escobar, Obregón and Escobar del Rey 14 ). Thyroid hormones are also involved in the regulation of BMR and macronutrient metabolism( Reference Oppenheimer, Schwartz and Mariash 15 ).
During pregnancy, iodine requirements increase substantially because of increased thyroid hormone synthesis and demand. Due to the trend of insufficient iodine intake in pregnancy, the WHO has increased the daily iodine intake recommendation for pregnancy to 250 µg( 1 ). In most countries, this intake is not provided by food sources, even more so because of suggestions by the US Food and Drug Administration and Environmental Protection Agency that pregnant women and nursing mothers lower their intakes of fish and shellfish that contain low levels of mercury and avoid products that have high levels( Reference Solan and Lindow 16 ). In addition, in countries such as Latvia, where the consumption of iodized salt is voluntary, the household coverage of iodized salt varies widely (i.e. lower dietary salt intake and decreased proportion of foods fortified with iodine).
Recently, a meta-analysis from the UK observed that iodine supplementation improves maternal thyroid indices and parameters of cognitive function, even in marginal iodine-deficient areas( Reference Taylor, Okosieme and Dayan 17 ). Nevertheless, in clinical practice, iodine supplementation is associated with concerns that sharp increases in iodine intake, even in marginally iodine-deficient populations, may increase the prevalence of thyroperoxidase antibodies (TPO-Ab)( Reference Teng, Shan and Teng 18 ). Therefore, the aim of the present nationally representative study was to assess the iodine status among pregnant women in Latvia and study the possible associations between TPO-Ab, thyroid-stimulating hormone (TSH) and free throxine (fT4) and iodine supplementation.
Materials and methods
To obtain results that could be extrapolated to all pregnant women in Latvia, a twenty-cluster survey was performed in all regions of Latvia during the spring and autumn seasons in 2013, with at least twenty women per cluster. Sample size calculation was based on an estimated 70·4 % prevalence of urinary iodine concentration (UIC) <150 µg/l, a 95 % confidence interval, a design effect of 2 and an absolute precision 5 %, resulting in a total sample size of 642 women. This prevalence figure was based on a population survey among Latvian schoolchildren( Reference Konrade, Dambrova and Makrecka 19 ). All included women were Caucasian. After receiving a detailed explanation from the study midwife, the study participants independently completed a questionnaire concerning the use of iodine supplements and the consumption of seafood, dairy products and iodized household salt, as well as smoking history, previous thyroid diseases and parity. From each participant a single blood sample and a portion of urine were collected for laboratory tests.
Laboratory measurements
An assay to detect urinary iodine using ammonium persulfate was adapted from methods described previously( Reference Dunn, Crutchfield and Gutekunst 20 ). Absorption measurements were obtained at 405 nm after 30 min incubation at room temperature using a μQuant™ Microplate Spectrophotometer (BioTek Instruments, Winooski, VT, USA). The urinary creatinine concentration was measured using the Jaffe method( Reference Jaffe 21 ) with the intention that iodine concentration adjusted for creatinine concentration (iodine/Cr) could be calculated. Creatinine-standardized UIC is a more reliable method of iodine excretion than random spot UIC measurement since there is a great day-to-day variability in water intake( Reference Soldin 22 ). Blood samples were sent to the E. Gulbis Laboratory (Riga, Latvia), which operates according to EN ISO 15189:2008 standard. TSH, fT4 and TPO-Ab were measured by chemiluminescence immunoassay (Siemens, Malvern, PA,USA) performed on an Advia Centaur XP (Siemens) analyser. We used the reference range recommended by the American Thyroid Association( Reference Stagnaro-Green, Abalovich and Alexander 23 ) for serum TSH (first trimester, 0·1–2·5 mIU/l; second trimester, 0·2–3·0 mIU/l; third trimester, 0·3–3·0 mIU/l) and TPO-Ab (0–64 IU/ml). Because the issue of TPO-Ab positivity in relation to iodine supplementation is clinically relevant, only pregnant women without known pre-existing thyroid disease were selected for the statistical analysis. Out of 665 women without prior thyroid disease, TPO-Ab was measured in 496 (number of women tested in the first, second and third trimesters were 122, 182 and 192, respectively).
Statistical analyses
The results are expressed as medians with interquartile range (IQR) and as percentage of participants in the study. The Mann–Whitney U test or Kruskal–Wallis test was used to compare UIC between two or more subgroups, respectively. Differences between prevalence estimates were tested using the χ 2 test or Fisher’s exact test, as appropriate. Spearman’s rank correlation analysis was performed. P values below 0·05 were considered statistically significant. Multivariate linear regression was used to analyse the factors associated with logarithmically transformed creatinine-standardized UIC, and multivariate logistic regression was used to analyse the factors associated with elevated TPO-Ab (>60 IU/ml). Only women with complete records were included in multivariate regression analyses. The data were analysed using the IBM SPSS 19·0 statistical software package and the Confidence Interval Analysis software.
Results
Of the 829 pregnant women enrolled in the current study, 739 women completed the study questionnaire (Table 1). Of the 162 women in the first trimester of pregnancy at the time of evaluation, 50·3 % (n 81) reported regular use of any dietary supplements irrespective of iodine content. The percentage of women using supplements was 63·6 % (n 178) in the second trimester and 65·5 % (n 190) in the third trimester; both values were significantly greater than those of women in the first trimester who used supplements (P=0·006 and P=0·002, respectively). Only 3·1 % (n 5) of women used supplements containing ≥150 μg iodine in the first trimester. The percentage of women using these supplements was also significantly greater in the second trimester (P=0·008), reaching 10·0 % (n 28), but the percentage was not significantly greater in the third trimester (5·9 % (n 17)) compared with the first trimester (P=0·193).
Table 1 Characteristics of the study population: nationally representative sample of pregnant women (n 739), Latvia, 2013
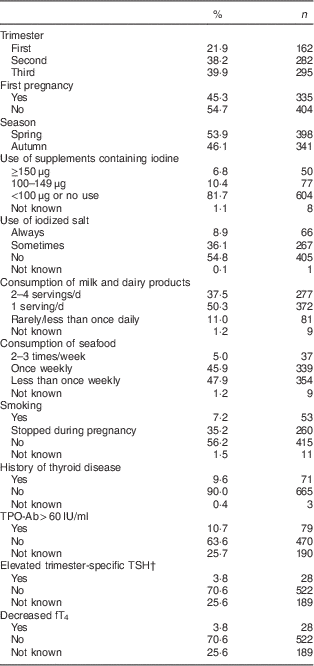
TPO-Ab, thyroperoxidase antibodies; TSH, thyroid-stimulating hormone; fT4, free thyroxine.
† According to the guidelines of the American Thyroid Association: first trimester, >2·5 mIU/l; second trimester, >3·0 mIU/l; third trimester, >3·0 mIU/l.
The UIC was measured in 696 pregnant women (Fig. 1). The median UIC among pregnant women was 80·8 (IQR 46·1–130·6) μg/g Cr (69·4 (IQR 53·9–92·6) μg/l); the 0·5 % percentile was 3·0 μg/g Cr and the 99·5 % percentile was 621·4 μg/g Cr. Of these women, 81 % had UIC measurements below 150 μg/g Cr, 62 % had UIC below 100 μg/g Cr and 28 % had UIC below 50 μg/g Cr. Only 7 % of the pregnant women exceeded a UIC of 249·9 μg/g Cr and 1 % (n 7) had UIC above 500 μg/g Cr. The median standardized UIC in the first trimester was significantly lower than that in the second and third trimesters (P<0·001 for both; Fig. 2(a) and (b)). A significant negative correlation between the gestational week and fT4 concentration was observed (Spearman’s ρ=−0·367, P<0·001; data not shown). The median fT4 concentration was 14·4 pmol/l in the first trimester, 13·1 pmol/l in the second trimester and 12·5 pmol/l in the third trimester (P<0·001). Hypothyroxinaemia (as defined by fT4 concentration below 10·3 pmol/l) was not detected in the first trimester but reached the prevalence of 2·5 % in the second trimester and 10·7 % in the third trimester (P<0·001). In the setting of mild maternal iodine deficiency, hypothyroxinaemia was not associated with UIC and TPO-Ab.
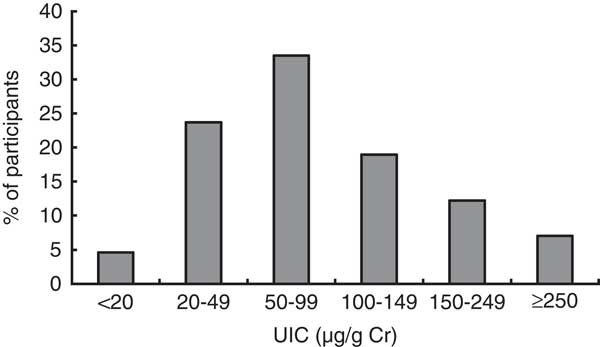
Fig. 1 Frequency distribution of iodine excretion among a nationally representative sample of pregnant women (n 696), Latvia, 2013 (UIC, urinary iodine concentration; Cr, creatinine)
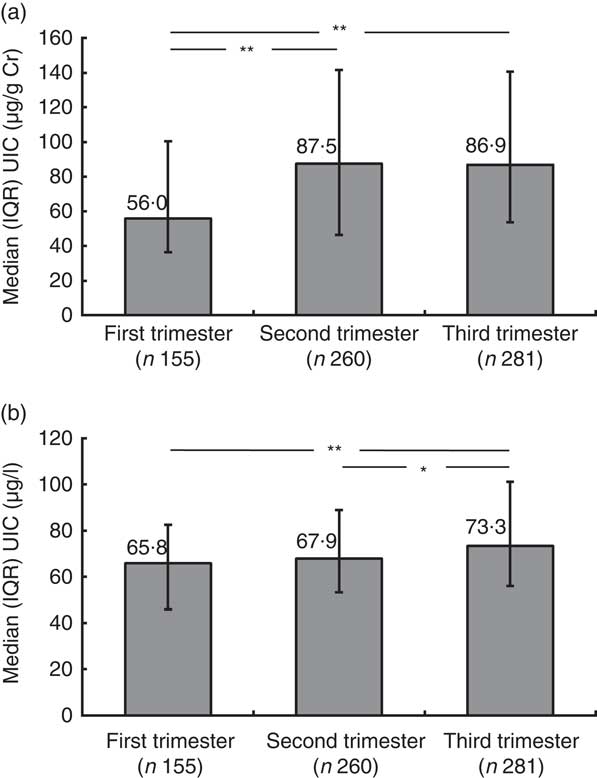
Fig. 2 Median iodine excretion during pregnancy trimesters among a nationally representative sample of pregnant women (n 696), Latvia, 2013: (a) UIC (μg/g Cr); (b) UIC (μg/l). Values are medians with interquartile ranges represented by vertical lines; horizontal lines linking bars indicate statistically significant (*P<0·05; **P<0·001) differences between the groups (UIC, urinary iodine concentration; Cr, creatinine; IQR, interquartile range)
As shown in Table 2, the median UIC was 80·3 (45·8–127·9) μg/g Cr in the group of women who did not use iodine-containing supplements or used supplements with an iodine content of <100 μg. A non-significantly higher median UIC of 86·2 μg/g Cr was detected in women using supplements with 100–149 μg iodine and 96·2 μg/g Cr with ≥150 μg iodine (P=0·471). Women who regularly used iodized salt had a non-significantly higher median UIC than did those who used regular salt, at 86·2 and 79·9 μg/g Cr, respectively (P=0·234). Women who reported only rarely consuming milk products had a median UIC of 65·2 μg/g Cr, which was significantly lower than that in the group of women consuming approximately three servings of milk products daily (87·6 μg/g Cr; P=0·002) and the women consuming one portion of milk products daily (80·1 μg/g Cr; P=0·047). The difference between the latter two groups was non-significant (P=0·076).
Table 2 UIC (μg/g Cr) according to the participants’ characteristics and dietary habits; nationally representative sample of pregnant women (n 696), Latvia, 2013
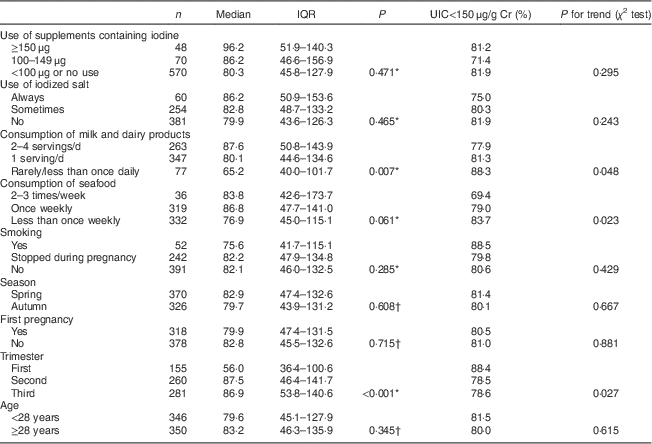
UIC, urinary iodine concentration; Cr, creatinine; IQR, interquartile range.
* P value from Kruskal–Wallis test.
† P value from Mann–Whitney U test.
Multivariate linear regression analysis was performed to assess the demographic and dietary factors associated with creatinine-standardized UIC in the present study. As shown in Table 3, the women’s age, gestational age, milk consumption (at least one serving daily) and seafood consumption (once weekly) were independently associated with a higher UIC, but parity was associated with a lower UIC.
Table 3 The association between dietary factors and participants’ characteristics and logarithmically transformed UIC (μg/g Cr) in multiple linear regression analysis; nationally representative sample of pregnant women (n 683), Latvia, 2013
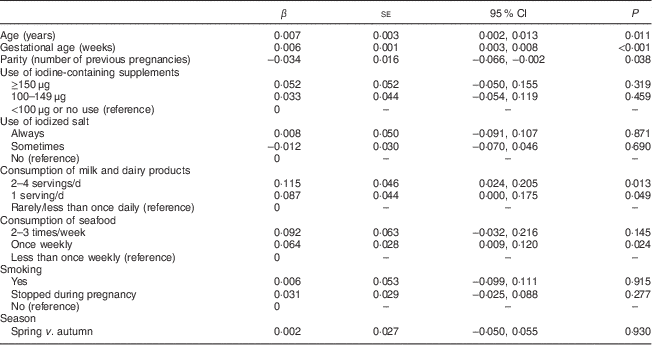
UIC, urinary iodine concentration; Cr, creatinine.
Because the issue of TPO-Ab positivity in relation to iodine supplementation is clinically relevant, only pregnant women without known pre-existing thyroid disease were selected for the statistical analysis (n 496). The prevalence of TPO-Ab concentration above 60 U/ml in spring was 17·3 %, which was significantly higher than that in autumn, 8·9 % (P=0·005; Table 4). The prevalence of TPO-Ab concentration above the reference range in the group of women with TSH concentration within the trimester-specific range was 12·5 %, but it reached a higher prevalence of 21·7 % in women with an elevated trimester-specific TSH concentration, although the difference was not statistically significant (P=0·201). No association between higher prevalence of elevated TPO-Ab concentration and iodine content in supplements was detected in our study (P for trend=0·565). Multivariate logistic regression analysis indicated that the odds of having an elevated TPO-Ab concentration were almost two times greater in spring than in autumn (OR=1·97; 95 % CI 1·09, 3·54) and nearly three times lower in women consuming one serving of dairy products daily than in women rarely consuming dairy products (OR=0·38; 95 % CI 0·17, 0·85). The odds of having a TPO-Ab concentration >60 U/ml were not increased by using supplements containing iodine, consuming iodized salt or fish, age, gestational age, parity or smoking habits.
Table 4 The association between patients’ characteristics and dietary factors and the prevalence of elevated TPO-Ab (>60 U/ml) during pregnancy in multiple logistic regression analysis; nationally representative sample of pregnant women (n 496), Latvia, 2013
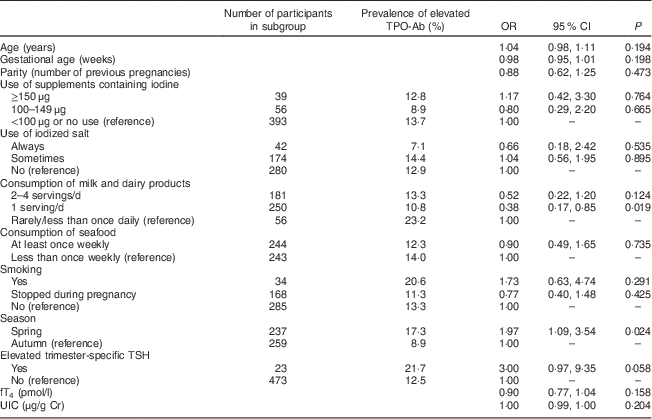
TPO-Ab, thyroperoxidase antibodies; TSH, thyroid-stimulating hormone; fT4, free thyroxine; UIC, urinary iodine concentration; Cr, creatinine.
Discussion
A previous cross-sectional school-based survey in Latvia showed that the median inter-seasonal creatinine-standardized UIC in schoolchildren was 107·3 (IQR 69·1–161·7) μg/g Cr( Reference Konrade, Dambrova and Makrecka 19 ). Despite the fact that the overall median UIC in schoolchildren remained within the low-normal range, a large percentage of the Latvian population had suboptimal iodine intake and were found to be iodine-deficient. This result occurs for example in pregnancy, when maternal needs for thyroid hormone increase due to the transfer of iodine and thyroid hormone to the fetus and the increase in maternal renal iodine clearance( Reference Zimmermann, Jooste and Pandav 24 ). Without a mandatory salt fortification programme or the iodization of cattle food (there are no nationally representative data on iodine content in milk available) and with a decreasing proportion of food being fortified with iodine, an inevitable outcome is iodine deficiency. This outcome is also illustrated by the results of the present study of pregnant women in Latvia, where the median UIC of 80·8 µg/g Cr indicates insufficient iodine intake according to WHO criteria( 1 ).
Several recent studies have suggested suboptimal iodine status in pregnant women living in areas with only partial coverage of iodized salt, such as Italy, the UK and the USA( Reference Marchioni, Fumarola and Calvanese 25 – Reference Hollowell, Staehling and Hannon 27 ). In a limited number of studies, long-term iodization before pregnancy showed more favourable effects on thyroid function than did short-term iodization and the early initiation of iodine supplements was more effective than beginning treatment in late pregnancy( Reference Moleti, Di Bella and Giorgianni 28 ). The present study also indicates that the median IUC was significantly higher in the second and third trimesters, which could be attributed to the intake of iodine-containing supplements. However, the intake of iodine supplements containing 150 µg iodine, as recommended by the American Thyroid Association( Reference Becker, Braverman and Delange 29 ), did not explain that association and also did not appear to be more effective in reducing the iodine deficiency during pregnancy. This might be attributed to the fact that in our study there was an insufficient number of women taking supplements containing ≥150 μg iodine to find the difference between subgroups statistically significant. Our data support the notion that the iodine deficiency was most likely already present before pregnancy.
We( Reference Konrade, Dambrova and Makrecka 19 ), along with others( Reference Arrizabalaga, Larrañaga and Espada 30 , Reference Moreno-Reyes, Carpentier and MacOurs 31 ), have reported seasonal UIC fluctuations in mild iodine-deficient regions of Europe. The data from the Latvian Neonatal TSH Registry also confirmed the seasonal differences in TSH levels in infants( Reference Konrade, Dambrova and Makrecka 19 ). However, in the present study, we did not find significant seasonal differences in IUC in pregnant women. This finding might result from the guidelines of the International Council for the Control of Iodine Deficiency Disorders and WHO suggesting that pregnant women intake approximately 40–50 % of iodine as dietary supplements, which masks the effect of seasonal pattern-dependent food sources, such as milk and milk products( Reference Als, Haldimann and Bürgi 32 ).
Surprisingly, contrary to the finding of no seasonality effect in the UIC values, our study reports that the prevalence of elevated TPO-Ab concentration was approximately two times higher in spring than in autumn. This finding is the first time that such an observation has been reported in pregnant women. Some type of seasonality effect has been observed in both the onset of type 1 diabetes mellitus and subclinical β-cell autoimmunity( Reference Samuelsson, Ludvigsson and Bottazzo 33 ), which is similar to the trends of seasonal respiratory tract infections (as the initial trigger). We have no information regarding previous history nor did we test for infection in our study population; therefore, the idea that infection is a contributing factor to TPO-Ab production remains speculative. In addition, a role for lower vitamin D levels is discussed in thyroid autoimmunity, but recent studies are inconsistent in this aspect( Reference Bozkurt, Karbek and Ucan 34 , Reference Effraimidis, Badenhoop and Tijssen 35 ).
In the present study, we did not observe any association between UIC and elevated TPO-Ab values. When more than 80 % of the pregnant women have UIC lower than 150 μg/g Cr, iodine supplementation is not expected to trigger thyroid autoimmunity, a result that has been noted in populations with UIC that were approximately three times greater( Reference Guan, Li and Li 36 ). In countries such as Latvia, excessive iodine intake is unlikely, even when regularly consuming iodized salt at the WHO recommended amount of 5 g/d( 37 ) and seafood once weekly during the pregnancy. Thus, in pregnant women natural dietary sources of iodine nutrition are less important.
In previous studies with randomized TPO-Ab+ women who received placebo or iodine treatment, the results were not statistically significantly different in terms of the rate of postpartum thyroid dysfunction( Reference Taylor, Okosieme and Dayan 17 ); therefore, the addition of 150 µg iodine daily does not pose a risk in a marginally iodine-deficient population such as Latvia. Taking into account the results of the present study, national recommendations for iodine status optimization should be targeted at fetal neurocognitive prophylaxis, initiated by daily supplementation with 100 µg iodine and folic acid for women without known thyroid disease in the preconception period. At the first prenatal visit supplements containing 150 µg of iodine should be recommended. Because iodine may increase thyroid autoimmunity in man and this can occur at iodine amounts that are necessary to prevent iodine deficiency( Reference Latrofa, Fiore, Rago and Antonangeli 38 ), a universal autoimmune thyroid disease screening in the first trimester of pregnancy( Reference Dosiou, Barnes and Schwartz 39 ) should be also recommended.
In our study, we report UIC in μg/g Cr units. It is well known that during normal pregnancy, the glomerular filtration rate and renal plasma flow in women increase by 40–65 % and 50–85 %, respectively, and that tubular function and the processing of water and electrolytes are altered( Reference Jeyabalan and Conrad 40 ); in addition, changes in fluid intake may influence UIC results( Reference Andersen, Møller and Laurberg 41 ). Therefore, UIC standardization to urine creatinine minimizes the variation due to dilution and urine volume because both vary depending on different factors of pregnancy. In our opinion, it would be more accurate to assess iodine nutrition in pregnancy in μg/g Cr units and not μg/l (as the International Council for the Control of Iodine Deficiency Disorders recommends); however, we report both variants to facilitate comparisons with other studies.
There are several limitations to our study design. The cross-sectional design of the study poses some limitation on the interpretation of the observed associations of gestational age with UIC and fT4. As these are not repeated measurements over the course of pregnancy, but single measurements in different women at different stages of pregnancy, a temporal relationship cannot be confirmed. The use of iodized salt was self-reported; in several cases, the women replied positive when they had actually consumed sea salt, which in reality contains little iodine. In addition, no data are available on the overall iodine status in women of childbearing age in Latvia compared with the iodine status in pregnant women.
Conclusions
The median UIC indicates iodine deficiency in pregnant women in Latvia. Iodine supplementation (150 µg daily) and regular population-level UIC monitoring among pregnant women and women of reproductive age should be suggested to overcome iodine deficiency and to reach the recommended levels without inducing autoimmune processes.
Acknowledgements
Financial support: This study was supported by the Latvian Association of Endocrinology and the Latvian National Research Programme BIOMEDICINE. The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: I.Ko., D.R., V.P., A.L. and M.D. formulated the research question and designed the study; I.Ka., A.J., E.T., V.V., D.G. and M.M.-K. participated in sample collection and carried out biochemical measurements; I.S. and I.Ko. performed statistical analysis of the data; I.Ko. and M.D. drafted the manuscript; the article text was reviewed and approved by all co-authors. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the P. Stradins Clinical University Hospital Medical Ethics Committee of Latvia. Written informed consent was obtained from all subjects/patients.









