Introduction
Alveolar echinococcosis (AE) is a serious zoonotic parasitic disease caused by the larval stage (metacestode) of the fox tapeworm Echinococcus multilocularis (Kern et al., Reference Kern, Meezes da Silva, Akhan, Müllhaupt, Vizcaychipi, Budke and Vuitton2017). However, despite high parasite prevalences in European foxes and high environmental contamination with E. multilocularis eggs, the disease is recorded at very low incidences in humans. Interestingly, susceptibility to oncosphere invasion following the natural infection route (egg inoculation) and subsequent AE development widely varies among species. In Europe, cricetid species (Microtus arvalis, Arvicola scherman) and the muskrat (Ondatra zibethicus) have been found to be highly susceptible, since field studies reported AE with high prevalences. However, only a few confirmed cases of infected murid species have been documented in Europe, namely in Apodemus agrarius in Eastern Europe, and a house mouse (Mus musculus) and a wild rat (Rattus norvegicus) in France (reviewed by Romig et al., Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017). Furthermore, mouse and rat infections have occasionally been reported in Asia (Okamoto et al., Reference Okamoto, Fujita, Arikawa, Kurosawa, Oku and Kamiya1992; Iwaki et al., Reference Iwaki, Hatakeyama, Nonaka, Miyaji, Yokohata, Okamoto, Ooi, Oku and Kamiya1993; Romig et al., Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017). However, in this context it has to be considered that the ecological contribution of the different rodent species to the transmission of E. multilocularis is not only determined by their susceptibility to AE development, but also by the predation rate for the different rodent species by the definitive hosts (Beerli et al., Reference Beerli, Guerra, Baltrunaite, Deplazes and Hegglin2017).
Primary infection models (inoculation of eggs) in rodents are of high value to identify variations in susceptibility in wildlife species such as voles (M. arvalis, M. agrestis and Myodes glareolus) (Woolsey et al., Reference Woolsey, Jensen, Deplazes and Kapel2015a; Reference Woolsey, Bune, Jensen, Deplazes and Kapel2015b; Reference Woolsey, Jensen, Deplazes and Kapel2016) and cotton rats (Sigmodon hispidus) (Matsumoto et al., Reference Matsumoto, Kouguchi, Oku and Yagi2010). Considering laboratory rodents, several strains of mice (e.g. DBA/2, AKR/N, BALB/c and C57BL/6) showed rapid and progressive hepatic metacestode development (Matsumoto et al., Reference Matsumoto, Kouguchi, Oku and Yagi2010). Other rodent species, such as gerbils (Meriones unguiculatus), golden hamsters (Mesocricetus auratus) or Wistar rats (R. norvegicus), exhibit partial or complete resistance to oral inoculation with eggs (Webster and Cameron, Reference Webster and Cameron1961; Tang et al., Reference Tang, Wang, Peng, Tang and Chen2006; Reference Matsumoto, Kouguchi, Oku and Yagi2010; Armua-Fernandez et al., Reference Armua-Fernandez, Joekel, Schweiger, Eichenberger, Matsumoto and Deplazes2016, respectively).
As recently shown for primary infected Wistar rats that were immunosuppressed by dexamethasone (DXM), an altered immune status of the host influences the course of disease in otherwise resistant animals, leading to progressive hepatic metacestode development (Armua-Fernandez et al., Reference Armua-Fernandez, Joekel, Schweiger, Eichenberger, Matsumoto and Deplazes2016). Interestingly, comparable to the situation in rats, immunocompetent humans are considered rather resistant to infection after ingestion of eggs (Vuitton, Reference Vuitton2003; Gottstein et al., Reference Gottstein, Wang, Boubaker, Marinova, Spiliotis, Müller and Hemphill2015), whereas severe progression of AE development occurs in patients with impaired immunological status due to pregnancy (Yang et al., Reference Yang, Vuitton, Jones, Craig and McManus2005), HIV co-infection (Sailer et al., Reference Sailer, Soelder, Allerberger, Zaknun, Feichtinger and Gottstein1997) or drug-based immunosuppression (Bresson-Hadni et al., Reference Bresson-Hadni, Koch, Beurton, Vuitton, Bartholomot, Hrusovsky, Heyd, Lenys, Minello, Becker, Vanlemmens, Mantion and Miguet1999; Chauchet et al., Reference Chauchet, Grenouillet, Knapp, Richou, Delabrousse, Dentan, Millon, Di Martino, Contreras, Deconinck, Blagosklonov, Vuitton and Bresson-Hadni2014; Vuitton et al., Reference Vuitton, Demonmerot, Knapp, Richou, Grenouillet, Chauchet, Vuitton, Bresson-Hadni and Millon2015). A retrospective hospital study in Switzerland revealed that the rate of newly diagnosed AE cases has significantly increased in this centre by 10-fold in immunocompromised patients of various backgrounds during the last decade (2008–2017), compared to a 4-fold increase in immunocompetent patients (Lachenmayer et al., Reference Lachenmayer, Gebbers, Gottstein, Candinas and Beldi2019).
Experimental immunological investigations addressing susceptibility and resistance to E. multilocularis development have focused on studies with established liver metacestode infection or secondary infection (metacestode inoculation), associated with progressively established parasite-specific humoral and cell-mediated immune responses in a variety of mouse strains (reviewed by Gottstein et al., Reference Gottstein, Soboslay, Ortona, Wang, Siracusano and Vuitton2017). Besides, Nakao et al. (Reference Nakao, Kameda, Kouguchi, Matsumoto, Dang, Simon, Torigoe, Sasaki, Oku, Sugimoto, Agui and Yagi2011) supported the genetic basis of susceptibility in different mouse strains after oral E. multilocularis egg inoculation, by identifying quantitative trait loci which may affect and regulate the establishment and development of AE. Our previous experiments showed that in contrast to the resistance of rats after inoculation with E. multilocularis eggs, intraperitoneal injection of oncospheres and metacestode material leads to parasite development at the site of inoculation. This suggested that the resistance mechanisms in rats to oncosphere invasion differ from the protective mechanisms controlling the development of AE in the liver and abdomen. Further experiments with nude rats did not result in hepatic progression of the parasite, suggesting that T cells do not play a key role in the resistance to oncosphere invasion (Armua-Fernandez et al., Reference Armua-Fernandez, Joekel, Schweiger, Eichenberger, Matsumoto and Deplazes2016). On the other hand, treatment of rats at time of inoculation with E. multilocularis eggs with DXM, a glucocorticoid immunosuppressant that severely affects various innate and adaptive immune cell populations (Coutinho and Chapman, Reference Coutinho and Chapman2011), resulted in liver infections with metacestodes (Armua-Fernandez et al., Reference Armua-Fernandez, Joekel, Schweiger, Eichenberger, Matsumoto and Deplazes2016; Joekel and Deplazes, Reference Joekel and Deplazes2017). This led to the hypothesis that innate immune cells may primarily be responsible for defense against E. multilocularis oncosphere invasion.
Here, we examined the role of three innate immune cell populations that are abundant in the intestinal mucosa and/or part of the intestinal immunological barrier in this process: macrophages (MΦ), polymorphonuclear cells (PMN) (i.e. neutrophils, eosinophils and basophils) and natural killer (NK) cells. We investigated whether these innate immune cell populations are involved in the natural resistance against intestinal E. multilocularis oncosphere invasion, by experimental egg inoculation of Wistar rats that were depleted of one or several cell types.
Materials and methods
Parasite eggs
Echinococcus multilocularis eggs were obtained from naturally infected foxes during the regular Swiss hunting season in spring 2017. They were collected according to Joekel and Deplazes (Reference Joekel and Deplazes2017) and stored at 4°C in phosphate-buffered saline (PBS) with 100 IU penicillin, 100 μg streptomycin (Life Technologies, Switzerland) until use (approximately 8 weeks). Eggs resistant to sodium hypochlorite (SH) were considered as viable (Deplazes and Eckert, Reference Deplazes and Eckert1988). All experiments with E. multilocularis eggs were conducted under biosafety level 2 regulations approved by the Swiss Federal Office of Public Health (FOPH).
Experimental animals
The animal experiments described in this paper were authorized by the Cantonal Veterinary Office of Zurich, Switzerland (permission no. 017/16 and 018/16). Experiments were performed according to the Directive 2010/63/EU guidelines and relevant Swiss legislation. Female RccHan™:WIST (Wistar) rats (2–3 months of age, mean weight: 217 g) were purchased from Envigo (Netherlands), and female BALB/cJRj mice (2 months of age) were obtained from Janvier Labs (France). Mice represented in vivo controls for infectivity of the utilized eggs and were inoculated alongside the rats in each experiment.
Experimental design of in vivo experiments
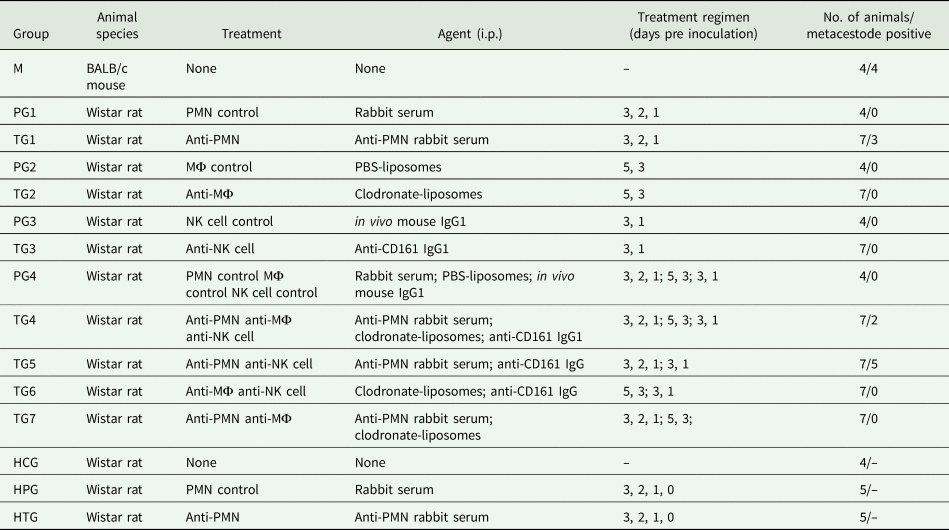
The design of the experiments is summarized in Table 1. Rats were divided into 11 groups: seven selectively depleted treatment groups (TG1–7; n = 7 per group) and four immunocompetent placebo treated groups (PG1–4; n = 4 per group). All treatments were administered intraperitoneally. Each rat from groups TG1, TG4, TG5 and TG7 received 100 μL anti-rat PMN rabbit serum (diluted 1/10 in sterile PBS; Acris Antibody GmbH) according to a protocol of Friedrich et al. (Reference Friedrich, Flores, Muller, Bi, Peerschke and Sehba2011) and each rat from groups PG1 and PG4 received 100 μL rabbit serum (diluted 1/10 in sterile PBS; Gibco™ Fisher Scientific) 3, 2 and 1 days prior to parasite inoculation. Each rat from groups TG2, TG4, TG6 and TG7 received 1.2 mL clodronate-liposomes (5 mg mL−1; ClodronateLiposomes.com), and each rat from groups PG2 and PG4 received 1.2 mL PBS-liposomes (ClodronateLiposomes.com) 5 and 3 days prior to parasite inoculation (protocol according to the manufacturer's recommendations). Each rat from groups TG3, TG4, TG5 and TG6 received 100 μL (300 μg) of mouse anti-rat CD161 (anti-NK cell) monoclonal antibody [immunoglobulin g (IgG) isotype; kindly provided by Bernard Vanhove, University of Nantes, France] according to Schwarzkopff et al. (Reference Schwartzkopff, Schlereth, Berger, Bredow, Birnbaum, Böhringer and Reinhard2010), and each rat from groups PG3 and PG4 received 100 μL (300 μg) of mouse anti-rat in vivo isotype control antibody (IgG1 isotype; LuBio Science GmbH) 3 and 1 days prior to parasite inoculation. All animals were orally inoculated with 1000 SH-resistant E. multilocularis eggs and euthanized 70 days after inoculation.
Table 1. Experimental design and results. Establishment of Echinococcus multilocularis metacestodes in Wistar rats that underwent the indicated treatment regimens. The inoculation dose (p.o.) for each animal consisted of 1000 SH-resistant E. multilocularis eggs; autopsy was done 10 weeks p.i.
In addition, 14 rats [four non-treated, non-infected rats (histology control group ‘HCG’ in Table 1), five rats treated with unspecific rabbit serum (histology placebo group ‘HPG’ in Table 1) and five rats treated with anti-PMN rabbit serum (histology treatment group ‘HTG’ in Table 1)] were orally inoculated with 1000 SH-resistant E. multilocularis eggs and euthanized approximately 16–17 h after egg inoculation.
Flow cytometry
To analyse the cell counts at the day of oral inoculation with E. multilocularis eggs, approximately 100 μL of blood was taken prior inoculation. Erythrocytes were lysed using ACK lysing buffer (150 mm NH4Cl, 10 mm KHCO3 and 0.1 mm Na2EDTA). For flow cytometric analysis, blood cells were subsequently stained with mouse anti-CD161 (anti-NK cell, clone 3.2.3, kindly provided by Bernard Vanhove, University of Nantes) and mouse anti-rat granulocyte HIS48 (Life Technologies) in ice-cold PBS supplemented with 1% FCS, 5 mm EDTA and 0.02% NaN3. LIVE/DEAD Near-IR stain (Life Technologies) was used for exclusion of dead cells. For intracellular staining, blood cells were fixed and permeabilized using BD Cytofix/Cytoperm reagent (BD Bioscience) and subsequently incubated in Perm/Wash buffer (BD Bioscience) containing mouse anti-rat CD68 (clone ED1, BioRad). Cells were acquired on a FACS Gallios (Beckman Coulter) and the data were analysed using FlowJo software (Tristar) following the guidelines for the use of flow cytometry and cell sorting in immunological studies (Cossarizza et al., Reference Cossarizza, Chang and Radbruch2019). In all experiments, the cells were pre-gated on viable, single cells for analysis.
Post-mortem examinations
Necropsy was performed on all rats immediately after CO2-euthanasia. From animals sacrificed 16–17 h after egg inoculation, the small intestines were dissected and processed for histological examination. In rats sacrificed at day 70, the livers were grossly examined and photographed.
Histological and immunohistological examinations, morphometric analysis
After fixation, the small intestine of the rats sacrificed 16–17 h after egg inoculation was dissected into the following segments: (a) duodenum (D): the proximal 8% of the intestine's length; (b) jejunum: the middle 90% of the intestine's length (divided into three segments of equal length; J1–J3) and (c) ileum (I): the distal 2% of the intestine's length. Each segment was then processed as a roll to prepare cross-sections of the entire intestine, following previous published protocols (Bialkowska et al., Reference Bialkowska, Ghaleb, Nandan and Yang2016). The tissue was fixed in 10% buffered formalin for 48 h and routinely paraffin wax embedded. Consecutive sections (5 μm) were prepared and routinely stained with haematoxylin-eosin (HE) or subjected to immunohistological staining. Immunohistology was carried out to detect neutrophils (myeloperoxidase (MPO)-positive) and MΦ (CD68-positive, MPO-positive) in the intestinal segments J1–J3, applying the horseradish peroxidase (HRP) method. Briefly, after deparaffinization, for the detection of MPO, staining was undertaken with an autostainer (Discovery XT, Ventana), using EDTA buffer (pH 9.0, 20 min at 98°C) for antigen retrieval, followed by incubation with the primary antibody (rabbit anti-MPO; ThermoFisher) in dilution buffer (1:30; Roche) for 1 h at 37°C, followed by the secondary antibody (donkey anti-rabbit IgG; Jackson ImmunoResearch) and the detection kit (DAB-Map-Kit; Roche). For the detection of CD68, the slides were incubated with the primary antibody (mouse anti-rat CD68; BioRad) and diluted in dilution buffer (1:500; Dako) for 1 h at room temperature (RT) in an autostainer (Dako), followed by blocking of endogenous peroxidase (peroxidase block, S2023, Dako) for 10 min at RT and incubation with the secondary antibody and detection system (MACH4 HRP Polymer, Biocare Medical) according to the manufacturer's recommendations. The antibody reaction was visualized with 3,3′-diaminobenzidin and sections counterstained with haemalaun.
A morphometric analysis was attempted to assess the small intestines for any quantitative difference in the number of MPO-positive cells present in the mucosa, as an indirect means to identify the number of neutrophils. Histological sections were scanned using a digital slide scanner (NanoZoomer-XR C12000; Hamamatsu, Japan) and the morphometric evaluation was undertaken with the computer program VIS (Visiopharm Integrator System, Version 2019.02.2.6239, Visiopharm, Hoersholm, Denmark). An application (APP) with a two-step process was developed. For each selected jejunal segment (J1–J3), an approximately 0.5 cm long random manual annotation of regions of interest (ROIs) was introduced. A first image segmentation to select the intestinal tissue was performed using the threshold classification method. Subsequently, the selected intestinal tissue was outlined as ROI (J1, J2 and J3). In the second step, MPO-positive cells (with brown cytoplasmic reaction) within the ROIs were selected with the threshold classification method. The results were expressed as the area of positive cells in each ROI (area of positive cells/total area).
Echinococcus multilocularis antigen preparation for in vitro experiments
Preparation of AO (activated oncospheres), sAO (somatic antigen of AO) and AO E/S (excretory/secretory antigen of AO)
Echinococcus multilocularis oncospheres were processed by artificial enzymatic activation according to the protocol of Deplazes and Gottstein (Reference Deplazes and Gottstein1991). After addition of pancreatin, pig bile and 2% NaHCO3, the tube was capped, vigorously shaken repeatedly and incubated in a water bath at 39°C for 15 min (until >80% of oncospheres showed active movement). Subsequently, the oncospheres were counted in a Neubauer counting chamber. After washing oncospheres three times with sterile PBS by centrifugation, 10 μL HBSS per 1000 AOs were added to the tube and the oncospheres were incubated for 2 h at 37°C and 5% CO2 to produce E/S antigens. After incubation, the tube was centrifuged at 10 000 g for 5 min and the supernatant was stored at −80°C until use (referred to as AO E/S antigen). The pellet was lyophilized (VirTis freeze dryer), crushed with a pestle and HBSS-buffer (10 μL per 1000 AOs) added to the homogenate. The suspension was frozen at −80°C until use (referred to as somatic (s)AO antigen).
Isolation of neutrophils from bone marrow
Neutrophils were separated from the femoral and tibial bone marrow of the rats using density gradient centrifugation. Briefly, 5 mL of Histopaque 1093 was laid upon 5 mL of Histopaque 1077 (both Sigma-Aldrich, Switzerland) in a 14 mL tube. Femur and tibia were dissected after CO2 euthanasia and opened at the ends. The bone marrow was flushed out with HBSS-buffer using a 1 mL syringe and a 25G cannula. The bone marrow suspension was laid on top of the gradients (1–3 mL) and the tube was centrifuged at 400 g for 30 min without break. The cloudy phase containing the neutrophils (second cloudy ring from top) was harvested, washed two times with HBSS and counted in a Neubauer chamber.
Quantification of neutrophil reactive oxygen species (ROS)
Rat neutrophils (2–4 × 105 cells) were added to each well of a 96-well microtitre plate and co-cultured in duplicates or triplicates with the same volume of either AO E/S (corresponding to E/S of 500 AOs), sAO (corresponding to somatic antigen of 500 AOs), AOs (n = 250 or 1000), PMA (as positive control; 5 μg well−1, Sigma-Aldrich) or HBSS (as negative control; containing CaCl2 and MgCl2, Sigma-Aldrich). Total ROS production was determined by adding luminol (100 μ m, Sigma-Aldrich). Chemiluminescence was measured with an Infinite 200 plate reader (Tecan) every 2 min upon co-cultivation at 37°C over a total time period of 120 min, starting immediately after co-cultivation and addition of the substrate.
Quantification of extracellular DNA as an indicator of NETosis
Rat neutrophils (2–4 × 105 cells) were cultured in 96-well tissue culture plates in HBSS (containing CaCl2 and MgCl2) and stimulated in duplicates or triplicates with either AO E/S (corresponding to E/S of 500 AOs), sAO (corresponding to somatic antigen of 500 AOs), AOs (n = 250 or 1000), zymosan (as positive control; 5 μg well−1, Sigma-Aldrich) or HBSS (as negative control; containing CaCl2 and MgCl2, Sigma-Aldrich) for 2.5 h at 37°C. After incubation, Sytox Green (160 nm, Invitrogen) was added to each well for the detection of extracellular DNA. Fluorescence was measured with an Infinite 200 plate reader (Tecan) (excitation at 485 nm and emission at 535 nm).
Results
Metacestode development in immune cell-depleted Wistar rats
To analyse whether selected immune cell populations are relevant for resistance against oncosphere invasion, PMNs, MΦ and/or NK cells were depleted from Wistar rats by treatment of the animals with anti-PMN rabbit serum, clodronate liposomes or anti-CD161 antibody, respectively, or combinations thereof prior to infection. Depletion efficiency was verified by flow cytometry at the day of inoculation with E. multilocularis eggs (Supplementary Fig. 1 and Table 1). Metacestode development in the liver was analysed 10 weeks after oral egg inoculation. All positive control BALB/cJRj mice (group M) developed metacestodes in the livers after inoculation, confirming the infectivity of the utilized eggs (Table 1). Placebo-treated Wistar rats (control groups PG1–4) showed no macroscopic evidence of infection. However, in all groups in which, inter alia, PMNs were depleted (groups TG1, TG4 and TG5) we observed active parasite development (alive metacestode tissue without protoscolex differentiation) in the livers (in total 10 of 21 rats), as shown in Fig. 1. Depletion of MΦ (group TG2) or NK cells (TG3) alone or in combination (TG6) and depletion of both MΦ and PMN (TG7), however, did not result in macroscopic signs of infection (Table 1). This suggests that PMN depletion results in loss of natural resistance to oncosphere migration and subsequent development in rats.
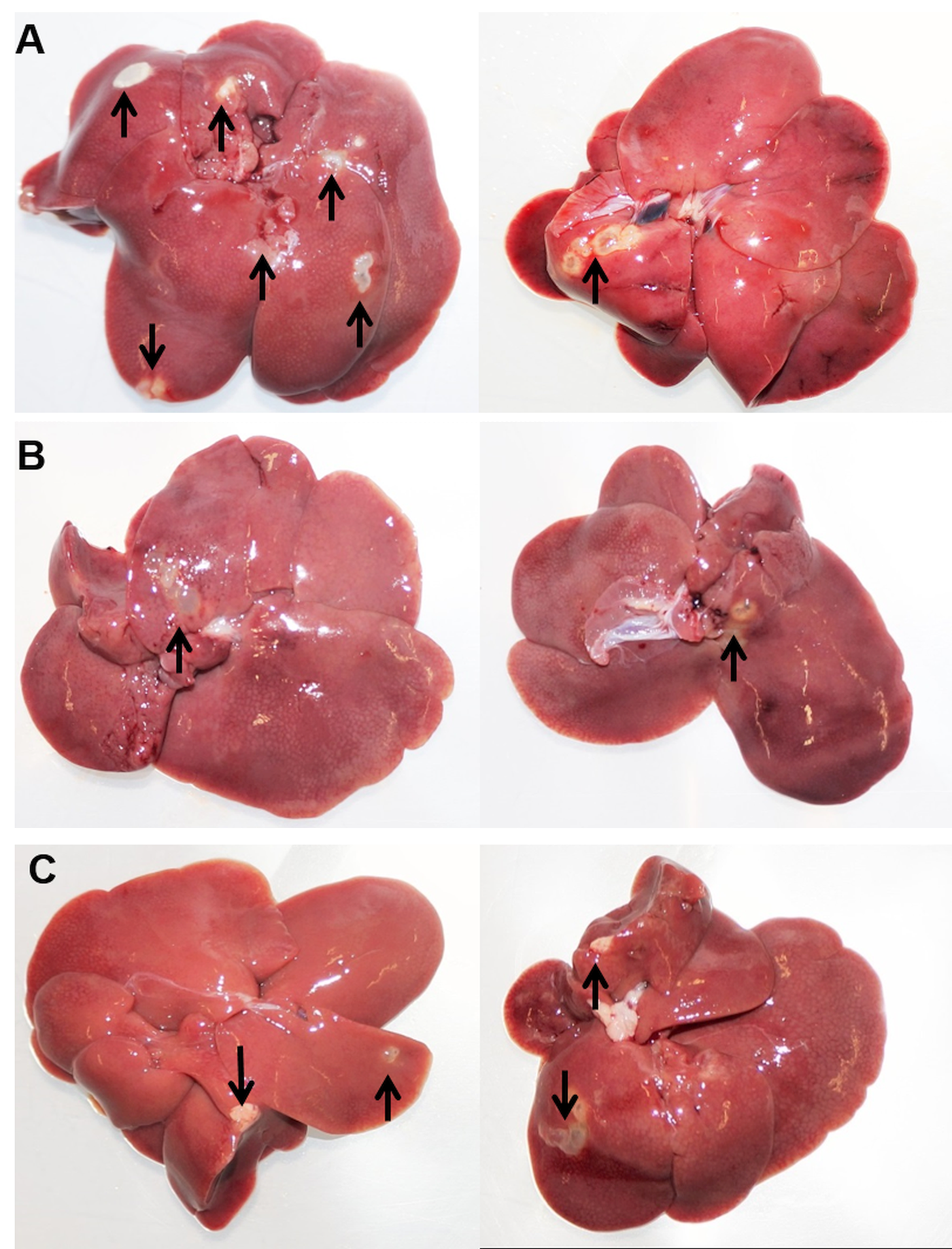
Fig. 1. Necropsy results at day 70. Echinococcus multilocularis-infected livers of group TG1 (PMN-depleted) (A), group TG4 (PMN-, MΦ- and NK cell-depleted) (B) and group TG5 (PMN- and NK cell-depleted) (C) at time of necropsy (study day 70). Two representative examples are shown for each group. Arrows show metacestode tissue.
Oral inoculation of rats with E. multilocularis eggs does not elicit any pathological changes in the small intestine
In all rats examined at 16–17 h post oral inoculation with E. multilocularis eggs and in non-infected controls, the small intestine did not exhibit any histological changes. The lamina epithelialis was intact, and the lamina propria showed mild diffuse infiltration by MΦ (CD68+), with lower lymphocytes, some plasma cells and few globular leucocytes/mast cells and eosinophils (Supplementary Fig. 2), representing the type and extent of leucocyte infiltration seen in the normal small intestine of rats (Nolte et al., Reference Nolte, Brander-Weber, Dangler, Deschl, Elwell, Greaves, Hailey, Leach, Pandiri, Rogers, Shackelford, Spencer, Tanaka and Ward2016). Neutrophils were not identified within the infiltrate in the non-treated, non-infected (control) rats, and in rats treated with anti-PMN rabbit serum (group HTG). In rats treated with unspecific rabbit serum prior to inoculation (group HPG), rare individual neutrophils (MPO-positive) were identified in the lamina propria surrounding the crypts (Fig. 2). In general, however, immunohistology suggested that MPO was expressed by 10–15% of infiltrating cells in the lamina epithelialis in all rats; apart from the rare individual neutrophils observed in the PG1 rats, these were mononuclear cells (i.e. MΦ). In order to identify any quantitative differences, a morphometric analysis was undertaken. However, this did not reveal any significant differences in the number of MPO-positive cells in the different groups (Supplementary Table 2), providing further evidence that oral inoculation of rats with E. multilocularis eggs did not elicit a detectable inflammatory response 16–17 h post egg inoculation in the small intestinal mucosa.

Fig. 2. Immunohistochemistry of rat intestines. Rats treated with unspecific rabbit serum (group PG1) and inoculated orally with E. multilocularis eggs 16–17 h post inoculation. Jejunum (J2). Crypts with surrounding lamina propria. (A) The lamina propria is diffusely infiltrated with a few MΦ and rare lymphocytes, eosinophils and globular leucocytes/mast cells. There is also one neutrophil (arrow). HE stain. (B) A proportion of mononuclear cells in the lamina propria exhibit MPO expression (MΦ). There is also one positive cell with a segmented nucleus (arrow), identified as a neutrophil. Immunohistology, haemalaun counterstain. Bars = 10 μm.
Ex vivo PMN stimulation revealed no ROS production or NETosis against oncospheres in vitro
Based on our in vivo results, we hypothesized that neutrophils are responsible for the natural resistance of rats against the parasite. To examine possible mechanisms by which neutrophils might block oncosphere invasion, we assessed the capacity of rat neutrophils to produce ROS and to undergo NETosis in response to E. multilocularis. Neutrophils were isolated from immunocompetent Wistar rats and exposed to E. multilocularis antigens (AO E/S, sAO and EmVF) in vitro. No significant induction of ROS (Table 2) or release of DNA into the extracellular space (as an indicator of NETosis, Table 3) could be detected, while positive control stimuli triggered a significant response. This indicates that the investigated strategies (ROS and NETosis) may not be relevant for the neutrophil-mediated defense against oncosphere invasion in resistant Wistar rats.
Table 2. Results of ROS activity in vitro. Rat neutrophils (2–4 × 105 cells) were co-cultured with either E. multilocularis AO E/S, sAO, AOs (n = 250 or 1000), PMA (positive control) or HBSS (negative control). Total ROS production was measured by chemiluminescence (CL) at 30, 60, 90 and 120 min. Mean = independent repeats (n) with 2–3 wells per condition each.
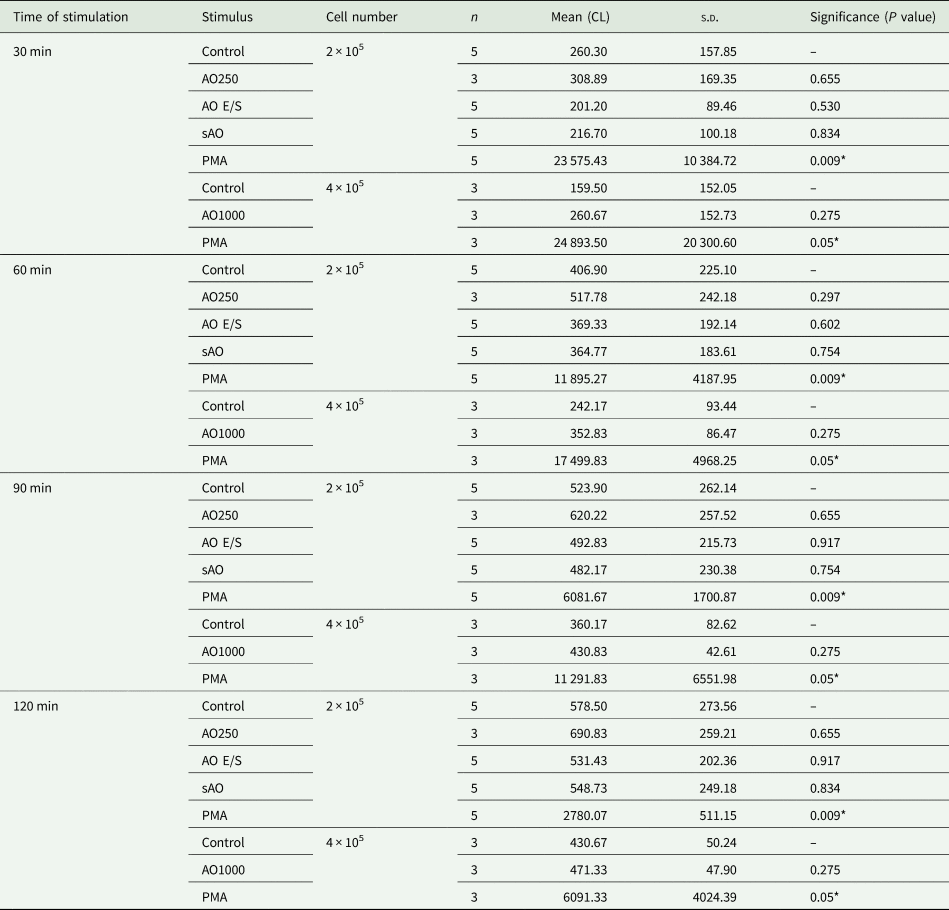
s.d., standard deviation of mean.
.
*P < 0.05.
Table 3. Results of extracellular DNA in vitro. Rat neutrophils (2–4 × 105 cells) were co-cultured with either AO E/S, sAO, AOs (n = 1000), zymosan (positive control) or HBSS (negative control). Total extracellular DNA was quantified by fluorescence after addition of Sytox green after 120 min of incubation. Mean = independent repeats (n) with 2–3 wells per condition each.
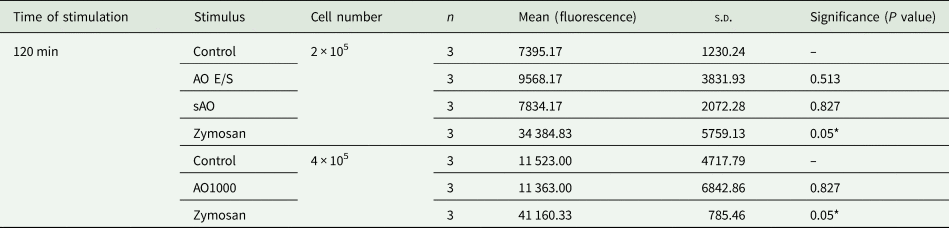
s.d., standard deviation of mean.
*P < 0.05.
Discussion
Varying susceptibility or resistance to E. multilocularis oncosphere migration and development among mammal species including humans seems to be based on hitherto unknown immunological mechanisms, with the exception that immunosuppressed individuals of several species show increased susceptibility for AE development after oral inoculation of eggs compared to immunocompetent ones, illustrated by previous case reports and animal experiments (Chauchet et al., Reference Chauchet, Grenouillet, Knapp, Richou, Delabrousse, Dentan, Millon, Di Martino, Contreras, Deconinck, Blagosklonov, Vuitton and Bresson-Hadni2014; Joekel and Deplazes, Reference Joekel and Deplazes2017). Our study aimed to investigate relevant innate immune cell populations and mechanisms behind the natural resistance of Wistar rats (a laboratory R. norvegicus strain). The main conclusion of our in vivo experiments is that PMN are key players in preventing oncosphere migration and development in E. multilocularis primary infected Wistar rats. Interestingly, NK cell and MΦ depletion alone were not associated with a reduction of this natural resistance. We further observed that MΦ seem to be important for PMN depletion in rats, as no PMN depletion was present when MΦ have initially been eliminated. The inability of neutrophil depletion by antibodies after depletion of MΦ by clodronate-liposomes was already described for mice, possibly due to the requirement of neutrophil opsonization by MΦ (Bruhn et al., Reference Bruhn, Dekitani, Nielsen, Pantapalangkoor and Spellberg2015). The reason why not all PMN-depleted animals became infected remains unclear. One possible explanation would be that the injected amount of anti-PMN antibodies may have been too low for some rats to induce a necessary level of depletion. Although flow cytometry data at the day of egg inoculation confirmed that PMN counts were significantly decreased in all depleted animals, the circulating pool is replenished rapidly, since millions of neutrophils are released per minute from the bone marrow into the blood (Summers et al., Reference Summers, Rankin, Condliffe, Singh, Peters and Chilvers2010). We administered the last anti-PMN injection 24 h before egg inoculation and must consider that oncospheres need several hours to pass the stomach with the ingesta and for their activation and migration through tissue and vessels in the small intestine. Therefore, it might be recommendable to include a further anti-PMN dose at the day of egg inoculation and even 1 day after, to ensure sufficient PMN depletion at the time of intestinal oncosphere invasion. In this context, it has to be considered that the reproducibility of such experiments might also be affected by differences in batches of polyclonal antisera. PMN are key players of the innate immune mechanisms and often described as first line defense against pathogen invasion after their recruitment to the site of infection by host- and/or pathogen-derived chemotactic stimuli (Yang et al., Reference Yang, Feng, Zhang, Lu and Zhao2017). Indeed, studies in mice demonstrated that neutrophils are key cells in the host defense against several fungal (Jensen et al., Reference Jensen, Warner and Balish1994; Romani et al., Reference Romani, Mencacci, Cenci, Sero, Bistoni and Puccetti1997), bacterial (Appelberg et al., Reference Appelberg, Castro and Silva1994; Conlan, Reference Conlan1997) and protozoan infections (Sayles and Johnson, Reference Sayles and Johnson1996). Furthermore, the importance of neutrophils for early protective immune responses against invading filarial third stage larvae of Litomosoides sigmodontis, mediated by the nucleotide-binding oligomerization domain-containing protein 2-dependent neutrophil recruitment, was demonstrated in a recent study (Ajendra et al., Reference Ajendra, Specht, Ziewer, Schiefer, Pfarr, Parčina, Kufer, Hoerauf and Hübner2016). Potential protective mechanisms by granulocytes during oncosphere migration have so far been rarely investigated, however, higher PMN counts were detected 4 days after oral inoculation with Taenia taeniaeformis eggs in moderate or highly resistant strains of mice compared to a susceptible mouse strain (Letonja and Hammerberg, Reference Letonja and Hammerberg1987). An older study found that ovine neutrophils were able to kill Taenia hydatigena oncospheres only in the presence of serum from infected sheep in vitro, whereas oncospheres were not affected by neutrophils and serum of non-infected sheep. The authors hypothesized that during initial contact of neutrophils and oncospheres (without the presence of specific antibodies or other mediators), neutrophils may not have any impact on oncosphere viability (Beardsell and Howell, Reference Beardsell and Howell1984). Another in vitro study conducted with murine eosinophils in response to oncospheral extract of the cestode Hymenolepis nana had similar results. Intestinal eosinophil numbers increased during H. nana infection in mice (Niwa and Miyazato, Reference Niwa and Miyazato1996a) and after their isolation from the lamina propria ROS was generated in response to oncospheral extract in vitro. However, radical generation from eosinophils of uninfected mice was negligible (Niwa and Miyazato, Reference Niwa and Miyazato1996b). This may explain the results of our in vitro experiments, since neutrophils of naïve rats did not produce significant ROS after incubation with E. multilocularis AOs or oncospheral products such as E/S or somatic antigen.
Neutrophil extracellular trap (NET) formation is a potent defense mechanism by neutrophils against parasite infections i.e. several protozoan species (Guimarães-Costa et al., Reference Guimarães-Costa, Nascimento, Froment, Soares, Morgado, Conceição-Silva and Saraiva2009; Abi Abdallah and Denkers, Reference Abi Abdallah and Denkers2012; Villagra-Blanco et al., Reference Villagra-Blanco, Silva, Muñoz-Caro, Yang, Li, Gärtner, Taubert, Zhang and Hermosilla2017) and nematode larvae, such as Strongyloides stercoralis (Bonne-Année et al., Reference Bonne-Année, Kerepesi, Hess, Wesolowski, Paumet, Lok, Nolan and Abraham2014), Haemonchus contortus (Muñoz-Caro et al., Reference Muñoz-Caro, Rubio, Silva, Magdowski, Gärtner, McNeilly, Taubert and Hermosilla2015) and Dirofilaria immitis (Muñoz-Caro et al., Reference Muñoz-Caro, Conejeros, Zhou, Pikhovych, Gärtner, Hermosilla, Kulke and Taubert2018). The inability of neutrophils to produce NETs in response to oncospheres, E/S or somatic antigen was surprising, since NETosis could have been an adequate mechanism to trap and immobilize oncospheres during migration in the intestinal mucosa or blood vessels, which has been shown for abovementioned motile nematode larvae. However, the results were in accordance with previous in vitro observations of the inability of bovine neutrophils to induce NETs (and also ROS) in response to (non-)AOs of Taenia saginata (Liliana Silva and Carlos Hermosilla, personal communication, University of Giessen, Germany). The results of the histopathological examination of the small intestines in our cohort of rats further support the in vitro findings, since there was no evidence of substantial neutrophil recruitment into the small intestinal mucosa in rats inoculated with E. multilocularis eggs without prior PMN depletion. Accordingly, we assume that another mechanism, other than ROS production or NETosis, of the defense repertoire of PMN might be considered during initial contact of oncospheres and PMN. Other cell types such as intestinal enterocytes could have potential impact on the initial priming and defense process of PMN during oncosphere invasion. It has been shown that depending on the luminal stimuli, intestinal epithelial cells regulate mucosal immune cells and vice versa, which determines control of disease and homoeostasis (reviewed by Turner, Reference Turner2009). However, the lack of any pathological changes in the lamina epithelialis, together with the lack of neutrophil influx into the mucosa suggest that the interaction of oncospheres with PMN may takes place only after the former have entered the blood stream.
Conclusion
We were able to significantly improve our rat model concerning animal welfare by applying a commercially available anti-PMN polyclonal serum instead of DXM (which induces up to 20% weight losses), since no adverse effects were recorded in any rat in this study. However, compared to DXM, the antibody-based PMN depletion was less effective, since several rats did not develop AE after oral inoculation with E. multilocularis eggs. Possible improvements for further experiments are discussed above. Rats are considered a good animal model to investigate E. multilocularis oncosphere invasion processes, since they are, comparable to the situation in humans, highly resistant. Therefore, the discovery of relevant mechanisms inhibiting parasite invasion and establishment in animal species considered as resistant may contribute to the understanding of the hitherto unknown mechanisms of susceptibility in humans. If we extrapolate the findings of our in vivo experiment to humans, several open questions come to mind: Is an increased susceptibility of humans to larval E. multilocularis invasion associated with an impaired granulocyte number or function? And, if so, is this increased susceptibility congenitally determined or due to (temporary) pharmacological immunosuppression e.g. drug treatment that incidentally induces agranulocytosis (Andersohn et al., Reference Andersohn, Konzen and Garbe2007)? In this context and with regards to increased AE susceptibility of HIV-infected patients, it has already been shown that neutropenia is common during HIV infection due to several reasons, inter alia viral cytopathic effects in haematopoietic tissue and the use of myelotoxic agents for treatment (Shi et al., Reference Shi, Sims, Hanna, Xie, Gulick, Zheng, Basson and Zhang2014).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182020002012
Acknowledgements
We highly appreciate Bernard Vanhove for kindly providing the mouse anti-rat CD161 (anti-NK cell) monoclonal antibody. The authors would like to thank Christina Lemberg for help with neutrophil functional assays. Furthermore, we wish to thank the Histology Laboratory, Institute of Veterinary Pathology, Vetsuisse Faculty Zurich, for excellent technical support.
Author contributions
Conceptualization and methodology: DEJ, PD; formal analysis: DEJ, SLL, JMR, AK; investigation: DEJ, SN, PAK, AK, JMR; writing – original draft: DEJ; writing – review and editing: PD, SN, SLL, AK, PAK, JMR; supervision: PD, SLL. All authors approved the final version of the manuscript before submission.
Financial support
This project was financed by the Forschungskredit of the University of Zurich (grant number FK-17-065) and by funds of the Institute of Parasitology, University of Zurich. The work in the LeibundGut-lab was supported by the Swiss National Science Foundation (grant number 310030_166206).
Conflict of interest
The authors declare that no competing interests exist.
Ethical standards
The animal experiments described in this paper were authorized by the Cantonal Veterinary Office of Zurich, Switzerland (permission no. 017/16 and 018/16). Experiments were performed according to the Directive 2010/63/EU guidelines and relevant Swiss legislation.








