Introduction
For piglets, the weaning transition from milk to a solid diet is characterised by a multitude of changes in the intestinal morphology and function, often accompanied by weaning-associated disorders(Reference Lallès, Boudry and Favier1). The various intestinal changes associated with weaning can, at least in part, be ascribed to the distinct lack of luminal nutrition arising as a consequence of reduced feed intake immediately after weaning(Reference McCracken, Spurlock and Roos2). Furthermore, several studies have shown that fasting or reduced feed intake may influence intestinal gene expression; for example, changes in intestinal gene expression have been reported for brush-border enzymes and nutrient transporters(Reference Ihara, Tsujikawa and Fujiyama3, Reference Ihara, Tsujikawa and Fujiyama4), and growth factors(Reference Ziegler, Almahfouz and Pedrini5, Reference Hoyt, Lund and Winesett6). Although coordinated alterations of intestinal gene expression were observed in piglets in response to weaning(Reference Wang, Chen and Li7), it is not always clear whether these changes are due to the weaning process per se or more related to associated reductions in feed intake.
Furthermore, specific micro- and macronutrients can have profound influence on intestinal gene expression, and, accordingly, on metabolism and on homeostasis(Reference Dauncey, White and Burton8, Reference Müller and Kersten9). The main agents through which nutrients influence gene expression are the transcription factors, which bind to specific DNA sequences of genes, thereby activating or inhibiting their transcription(Reference Mangelsdorf, Thummel and Beato10) and translating nutritional stimuli into changes in gene expression(Reference Schoonjans, Staels and Auwerx11). For example, a group of transcription factors, referred to as PPAR, are sensitive to dietary fatty acids(Reference Kersten, Desvergne and Wahli12), and some of them (PPAR-γ) are involved in the regulation of a number of inflammatory genes(Reference Jiang, Ting and Seed13, Reference Marx, Sukhova and Collins14), thereby leading to attenuation of inflammation. Thus, it seems to be possible that specific dietary components may beneficially influence response variables related, for example, to immune function and inflammation. In that way, diet could help to overcome problems associated to times of multiple stressors, as they occur, for example, during weaning. The present review includes two objectives. First, it aims to provide an insight on possible effects of reduced feed intake on intestinal gene expression, as it may occur during the weaning transition. This may help to understand intestinal dysfunctions and diseases related to the weaning period. Data from studies focusing on the effect of fasting or total parental nutrition (TPN) have been included, as effects of experimental fasting seem to correspond to the effects of immediate post-weaning anorexia(Reference McCracken, Spurlock and Roos2, Reference Pluske, Hampson and Williams15). Second, it is hypothesised under which conditions intestinal gene expression could be modulated by dietary means, thereby acting as a potential therapeutic tool. Therefore, examples are given of intestinal genes which may be altered in their expression as a consequence of supply with specific dietary nutrients, including carbohydrates, fats and proteins/amino acids. Reference is also made to studies investigating the effect of microbial fermentation metabolites on intestinal gene expression, as some of them, for example, SCFA(Reference Savage16, Reference Cousins17), have been shown to affect gene expression(Reference Davie18). As manipulation of the diet may be an appropriate way of changing the composition and activity of the gastrointestinal microflora(Reference Williams, Verstegen and Tamminga19, Reference Williams, Bosch and Boer20), this would open further perspectives to indirectly affect intestinal gene expression. The present review includes mainly data from pigs and rodents, but, if applicable, also from other species.
The weaning transition: a brief summary
For piglets, weaning involves complex psychological, social, environmental and dietary stresses that interfere with intestinal development and adaptation (Table 1). The immediate effect of weaning is a dramatic reduction in feed (energy) intake for up to 2 or 3 d(Reference Lallès, Boudry and Favier1) leading to undernutrition and a transient growth check(Reference Bruininx, Binnendijk and van der Peet-Schwering21). This temporary weaning anorexia results in alterations of intestinal integrity and appears to be one of the major aetiological factors in gut-associated disorders(Reference Pluske, Hampson and Williams15, Reference Hampson and Gyles22, Reference Spreeuwenberg23). Intestinal alterations often seen in piglets post-weaning include changes in villus–crypt architecture and in activities of many brush-border digestive enzymes. For example, marked villus atrophy ( − 45 to − 70 % of pre-weaning values), particularly in the proximal small intestine, has been repeatedly reported during the degenerative phase(Reference Pluske, Hampson and Williams15, Reference Spreeuwenberg23). Also, a reduction of lactase activity followed by increased maltase and sucrase activities immediately post-weaning have been consistently reported and may reflect maturation of intestinal function in relation to the weaning diet(Reference Pluske, Hampson and Williams15). These changes are thought to temporarily decrease the digestive and absorptive capacity of the small intestine. Furthermore, the transient alteration of intestinal integrity is characterised by increased mucosal permeability, and disturbed absorptive-secretory balance(Reference Boudry, Peron and Le Huërou-Luron24), probably also related to the weaning-associated fasting period imposed on piglets(Reference Goldstein, Hebiguchi and Luk25, Reference Carey, Hayden and Tucker26). In addition, a stimulation of pro-inflammatory cytokine gene expression in piglets post-weaning has also been reported(Reference Pié, Lallès and Blazy27). The pro-inflammatory cytokines IL-1β, IL-6 and TNF-α represent early non-specific mediators of inflammation, and are known to affect intestinal epithelial permeability and transport(Reference Lallès, Boudry and Favier1, Reference McKay and Baird28). Furthermore, increased T cell numbers and increased local expression of the matrix metalloproteinase stromelysin in the small intestine have been measured during post-weaning anorexia in piglets, and may contribute to intestinal inflammation(Reference McCracken, Spurlock and Roos2). In addition, the transition from milk to a solid diet leads to dramatic changes in the composition of the microbial population of the gastrointestinal tract (GIT) during the 7–14 d after weaning(Reference Hillman, Garnsworthy and Wiseman29). According to Ewing & Cole(Reference Ewing and Cole30), numbers of lactobacilli and other beneficial bacteria decrease in times of stress, as do their beneficial effects. This allows potential pathogens such as coliforms to increase. Franklin et al. (Reference Franklin, Mathew and Vickers31) found that lactobacilli populations in different GIT sections (jejunum, ileum, caecum) declined to lower levels in early-weaned pigs (17 d), compared with pigs weaned at 24 d. Accordingly, Janczyk et al. (Reference Janczyk, Pieper and Smidt32) found dramatic changes in the diversity of lactobacilli populations in the ileum around weaning. According to Konstantinov et al. (Reference Konstantinov, Awati and Williams33), two Lactobacillus species dominate in ileal samples of neonatal and unweaned piglets, but following weaning, these two lactobacilli significantly declined in numbers. Such weaning-associated changes in the intestinal microbiota can, in turn, also influence immune development(Reference Hooper and Gordon34). However, the molecular mechanisms underlying weaning anorexia and refeeding are not well established in piglets(Reference Mazurais, Romé and Cahu35). Recent studies which used microarray techniques have shown that weaning in piglets coincides with marked alterations in the expression of various intestinal genes, related to nutrient metabolism and/or cell proliferation and/or immune function(Reference Wang, Chen and Li7, Reference Niewold, Kerstens and Van der Meulen36). Such information would be relevant for improving the understanding of post-weaning gut disorders(Reference Mazurais, Romé and Cahu35).
Table 1 Weaning transition in young pigs (adapted from Lallès et al. (Reference Lallès, Boudry and Favier1))

Changes in intestinal gene expression due to reduced feed intake
Digestive function and nutrient metabolism
Several studies with rodents have investigated effects of changes in the nutritional status on intestinal gene expression, for example, of intestinal nutrient transporters. For example, short-term starvation in rats leads to a substantial increase in mRNA levels of the monosaccharide carrier by which fructose is absorbed (GLUT5)(Reference Castello, Guma and Sevilla37). Other nutrient transporters that are sensitive to alterations of the nutritional status are the oligopeptide transporters, such as the H+-dependent peptide transporter-1 (Pept1), which is responsible for absorption of the products of protein digestion from the intestinal lumen into the epithelial cell such as dipeptides and tripeptides(Reference Adibi38). It is accepted that Pept1 gene regulation is, amongst other factors, regulated by the diet(Reference Gilbert, Wong and Webb39). Short-term starvation over a period of 1 or 4 d in rats increases dipeptide absorption rates, paralleled by increases in Pept1 protein and mRNA levels(Reference Ogihara, Suzuki and Nagamachi40, Reference Thamotharan, Bawani and Zhou41). Similarly, increases in Pept1 mRNA levels were observed for rats which received 7 d TPN(Reference Howard, Goodlad and Walters42). This was also observed after 10 d of TPN, and an increased Pept1 protein density was noted(Reference Ihara, Tsujikawa and Fujiyama3). Consequently, fasting, both during short-term or prolonged periods, increases the Pept1 mRNA level, resulting in an increase in the population of the transporter in the brush-border membrane of the intestinal mucosa(Reference Adibi43). Increased expression and activity of Pept1 during feed deprivation or restriction may serve as a mechanism to compensate for a reduced mucosal surface area(Reference Gilbert, Wong and Webb39). According to Barbot et al. (Reference Barbot, Windsor and Rome44), the physiological significance of increased expression is to counterbalance the shortening of the absorptive surface during starvation. The latter can be even more important when there is an infection. For example, an enhanced level of Pept1 mRNA was found in suckling rats during acute cryptosporidiosis. This is an infection with Cryptosporidium parvum which frequently results in diarrhoea(Reference Barbot, Windsor and Rome44). The authors suggested that this transcriptional up-regulation plays an important role in the adaptation to malnutrition associated with cryptospiridiosis. Although the study of Barbot et al. (Reference Barbot, Windsor and Rome44) was performed with rats, one could also imagine a similar mechanism for pigs, to overcome malnutrition associated with diarrhoea. A mechanism for this induction of intestinal Pept1 during fasting was proposed by Shimakura et al. (Reference Shimakura, Terada and Saito45). They demonstrated in rats that the fasting-induced up-regulation of Pept1 expression is mediated via PPAR-α, a member of a family of ligand-activated nuclear receptors. The nuclear receptors are one of the largest groups of transcription factors, and are the most important group of nutrient sensors(Reference Mangelsdorf, Thummel and Beato10, Reference Francis, Fayard and Picard46, Reference Corthésy-Theulaz, Den Dunnen and Ferre47). PPAR-α is an important mediator of the hepatic response to fasting, and is believed to be a major regulator of lipid and glucose metabolism, allowing adaptation to the prevailing nutritional environment(Reference Dreyer, Krey and Keller48–Reference Kersten, Seydoux and Peters50). Shimakura et al. (Reference Shimakura, Terada and Saito45) hypothesised that increased Pept1 minimises the loss of N by efficient absorption of small-intestinal peptides derived from sloughing cells or secreted hormones. Furthermore, such an induction of Pept1 expression might be a preparation for adaptation when food is given again after fasting.
Also, the gene expression of intestinal transporters and phase I/II metabolic enzymes, which are required for adequate metabolism and excretion of various endogenous molecules, nutrients and xenobiotics, is affected by fasting(Reference Kaminsky and Zhang51, Reference Van den Bosch, Bünger and de Groot52). According to a study of Van den Bosch et al. (Reference Van den Bosch, Bünger and de Groot52) in the murine small intestine, 24 h of fasting resulted in the up-regulation of genes, for example, involved in the transport of energy-yielding molecules during glycogenolysis and during mitochondrial and peroxisomal oxidation of fatty acids. As some of the transporters and phase I and II genes are regulated in dependency of PPAR-α, it appears that the absorptive and detoxification capacity of the small intestine is altered during fasting, and PPAR-α mediates a part of these alterations(Reference Van den Bosch, Bünger and de Groot52).
Le Huërou-Luron et al. (Reference Le Huërou-Luron, Petersen, Hartmann and Ball53) studied in piglets small-intestinal changes due to weaning and found that a post-weaning increase of maltase activity paralleled that of maltase mRNA. In their study, maltase mRNA abundance and activity were 2·3-fold higher in 25 d weaned control than in 18 d-suckled piglets. Marion et al. (Reference Marion, Petersen and Romé54) investigated the effect of feed intake level after weaning on the activity of several brush-border enzymes and mRNA levels in early-weaned piglets (aged 7 d). In their study, however, only maltase showed weaning-induced increases in activity, which were closely correlated with corresponding mRNA levels. This suggests a mainly transcriptional regulation of maltase in response to weaning. However, changes in specific activities of brush-border enzymes during weaning may also reflect indirect effects resulting from a lower feed intake. Indeed, the most striking consequence of post-weaning anorexia is a rapid mucosal atrophy of the small intestine, associated with a reduction of villous height and increases in crypt depth which are generally associated with reductions in the specific activities of the brush-border enzymes lactase and sucrase(Reference Pluske, Hampson and Williams15, Reference Montagne, Boudry and Favier55). Alterations of intestinal integrity, however, were also observed in underfed piglets and piglets receiving TPN, when compared with their counterparts being fed ad libitum or receiving enteral nutrition, independently of the weaning process(Reference Carey, Hayden and Tucker26, Reference Park, Monaco and Donovan56). Mazurais et al. (Reference Mazurais, Romé and Cahu35) investigated the impact of fasting and refeeding on the piglet's jejunal transcriptome during the weaning period. In their study, 21-d-old sow-reared piglets were fasted for 2 d before slaughtering at the age of 23 d. Porcine arrays associated with real-time PCR were used to assess global gene expression changes in the jejunum of piglets at weaning. In this study, some genes associated with carbohydrate and lipid metabolism (6-phosphofructokinase, pyruvate kinase, triosephosphate isomerase, apoA-IV) were down-expressed in fasted and refed animals, while other genes involved in carbohydrate metabolism, for example, a member of carbonyl-reducing enzymes (short-chain dehydrogenase/reductase (SDR) family member 4)(Reference Matsunaga, Shintani and Hara57), were up-expressed in fasted piglets. The authors concluded from their study a major impact of weaning on the jejunal transcriptome. Although a direct correlation between transcriptomic regulation and functional impact is not always clearly to establish, more information on the underlying mechanisms of weaning-associated anorexia and subsequent refeeding would be needed for better understanding of weaning-associated gut disorders. Also, Wang et al. (Reference Wang, Chen and Li7) studied a wide range of genes by using microarray technology. They showed that early weaning in piglets coincides with a modulation of small-intestinal expression of several genes. In this study, feed intake was reduced by 36 % in weaned piglets as compared with suckling piglets. Reduced expression was shown in pigs aged 28 d weaned at age 21 d in comparison with age-matched suckling piglets, amongst others, for genes encoding for proteins related to protein and peptide degradation (for example, aminopeptidase A, cathepsin F), lipid metabolism (for example, acyl-CoA dehydrogenase, carnitine transporter 2) and intestinal transport (for example, apoA-IV precursor, fatty acid-binding protein, sodium- and chloride-dependent creatine transporter I). Down-regulation of these genes is expected to reduce intestinal transport and utilisation of dietary nutrients (particularly lipids and proteins). Other genes of lipid metabolism (for example, hydroxymethylglutaryl-CoA synthase, squalene epoxidase), but also of carbohydrate metabolism (galactoside 2-α-l-fucosyltransferase), showed enhanced expression. The authors concluded that early weaning would result in a decreased expression of genes related to nutrient utilisation.
Immune function
Wildhaber et al. (Reference Wildhaber, Yang and Spencer58) investigated the effect of completely reduced enteral nutrition on the function of intra-epithelial lymphocytes (IEL), as measured by IEL cytokine mRNA expression in mice. In this study, TPN-mice showed a decline in IL-2 and IL-10 expression, while IL-4, IL-6, interferon (IFN)-γ, transforming growth factor (TGF)-β1 and TNF-α were increased, as compared with mice receiving oral feeding, or receiving TPN plus oral feeding. This suggests that the lack of enteral nutrition is responsible for changes in intestinal immune function caused by TPN, and not the administration of the TPN solution itself. Alterations of IEL cytokine mRNA expression (for example, an increased IFN-γ mRNA expression) have also been shown by Kiristioglu & Teitelbaum(Reference Kiristioglu and Teitelbaum59) in mice receiving TPN. Changes in the expression of IEL-derived cytokines, particularly an up-regulation of IFN-γ expression, may be associated with a loss of epithelial barrier function in mice(Reference Yang, Kiristioglu and Fan60). This may contribute to problems often associated with TPN, such as an increased rate of bacterial translocation(Reference Alverdy, Aoys and Moss61). For the young animal, this would provide a link between weaning-associated anorexia, i.e. lack of luminal nutrition, and an enhanced susceptibility to intestinal infections observed during this period. A modified epithelial barrier function has also been observed in piglets resulting from weaning-associated anorexia, and might allow the passage of pathogens possibly leading to diarrhoea(Reference Lallès, Boudry and Favier1, Reference Boudry, Peron and Le Huërou-Luron24). Additionally, Fukatsu et al. (Reference Fukatsu, Kudsk and Zarzaur62) showed a decrease in IL-4 and IL-10 mRNA expression in lamina propria cells of mice receiving TPN compared with mice fed normal chow. These immunoregulatory cytokines are known as IgA-stimulating cytokines. As IgA is a critical component in mucosal immunity and barrier integrity, a decrease in expression of these cytokines would explain impaired mucosal immunity associated with the lack of enteral feeding(Reference Fukatsu, Kudsk and Zarzaur62, Reference Kramer, Sutherland and Bao63).
The process of weaning is associated with large differences in the number of genes expressed in the jejunal mucosa of piglets directly after weaning (aged 4 weeks) in comparison with older pigs (aged 12 weeks). The most notable contrast was the high number of immune-associated genes expressed in 4-week-old animals(Reference Niewold, Kerstens and Van der Meulen36). Furthermore, Wang et al. (Reference Wang, Chen and Li7) observed that early weaning modulated the expression of several genes in the small intestine of piglets (as compared with suckling piglets), mostly with an increased expression of genes that promote oxidative defence and immune activation. For example, enhanced expression was observed for genes responsible for immune function, for example, Igα chain C, Igκ chain C, Igλ chain and IgJ chain. These belong to polypeptide chains composing an antibody molecule. Also enhanced expression was shown for polymeric Ig receptor precursor and for lysozyme. The polymeric Ig receptor is a cell surface receptor of mucosal epithelial cells that mediates the transport of IgA and IgM through the epithelial cells into the intestinal lumen, where they function as inhibitors of bacterial/viral adherence and penetration of the underlying epithelium(Reference Abbas and Lichtman64), while lysozyme is an antimicrobial protein(Reference Chipman and Sharon65). An increased expression of glutathione transferase ω-1 could also be seen, suggesting the presence of oxidative stress in the small intestine of weaned piglets(Reference Wang, Chen and Li7, Reference Wu, Fang and Yang66). Indeed, intestinal oxidative stress has been associated with the initiation and propagation of intestinal pathologies, for example, ischaemic-reperfusion disorders(Reference Aw67, Reference Young, Fan and Mine68) for which intestinal lesions have been shown for newborn piglets similar to those seen in human newborns with necrotising enterocolitis(Reference Papparella, DeLuca and Oyer69, Reference Haase, Bigam and Nakonechny70). Furthermore, in piglets the pattern of pro-inflammatory cytokine gene expression in the intestine changes throughout weaning (weaned age 28 d), with an up-regulation of IL-1β, IL-6 and TNF-α mRNA during the first 2 d post-weaning. After 2 d post-weaning, the mRNA level of these cytokines returned to pre-weaning levels, with the exception of TNF-α mRNA level in the distal small intestine, which remained high(Reference Pié, Lallès and Blazy27). The cytokine IL-1β can be up-regulated in response to a wide range of specific and non-specific stimuli. In the study of Pié et al. (Reference Pié, Lallès and Blazy27), IL-1β expression increased in all segments investigated, suggesting that its up-regulation seems to be more related to the general stress associated with weaning rather than to a specific stimulation. Accordingly, in rats an initial increase of IL-1β expression in Peyer's patches was found at 19 d, and a further elevation at 21 d of age (the time of weaning), suggesting that this cytokine is involved in the differentiation and/or activation of epithelial cells at weaning(Reference Mengheri, Ciapponi and Vignolini71, Reference Schaeffer, Diab-Assef and Plateroti72). TNF-α, on the other hand, appears to be involved in the stimulation of Cl− secretion into the ileum(Reference Kandil, Berschneider and Argenzio73), suggesting that an increased expression of TNF-α might be implicated in the development of diarrhoea. Such early gene up-regulation of pro-inflammatory cytokines associated with weaning may contribute to early functional disorders, favouring the development of diarrhoea(Reference Pié, Lallès and Blazy27). In a further study of Pié et al. (Reference Pié, Awati and Vida74), the effect of enforced fasting (48 h) on mRNA content of pro-inflammatory cytokines in the ileum and colon of newly weaned piglets was investigated, and no correlation between the quantity of feed intake and the mRNA content of the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α could be observed at day 4 post-weaning. Other studies, however, reported that a lack of intestinal nutrient provision (i.e. parenteral feeding) favours the development of inflammation in the intestine of piglets(Reference Ganessunker, Gaskins and Zuckermann75, Reference Zijlstra, McCracken and Odle76).
Growth factors
Several nutrient-regulated intestinal growth factors, such as insulin-like growth factor (IGF)-I, are associated with fasting-induced atrophy and refeeding-induced intestinal regrowth(Reference Winesett, Ulshen and Hoyt77). Moreover, the administration of exogenous IGF-I has been noted to reverse the mucosal atrophy induced by TPN in rodents(Reference Dahly, Guo and Ney78). The IGF promote the proliferation and differentiation of cells in various organ systems, including intestinal epithelial cells(Reference Straus79). Their biological actions are mediated by binding to specific cell surface receptors(Reference Laburthe, Rouyer-Fessard and Gammeltoft80, Reference LeRoith, Werner and Beitner-Johnson81), and are directly regulated by IGF-binding proteins (IGFBP)(Reference Straus79, Reference Hardouin, Hossenlopp and Segovia82, Reference Cohick and Clemmons83). The IGF system, including IGF-I, IGF-II, the IGFBP and the IGF membrane-associated receptors, is highly sensitive to nutritional status(Reference Simmen, Badinga and Green84). In Table 2, some studies are summarised describing the effects of fasting/refeeding on growth factors. For example, fasting in rats decreased jejunal IGF-I mRNA levels and plasma IGF-I concentrations and induced intestinal atrophy(Reference Ziegler, Almahfouz and Pedrini5). These changes were reversed by refeeding. However, levels of IGF-I receptor and IGF-I receptor mRNA were not significantly altered during fasting, but they increased after refeeding. Such up-regulation of IGF-I and IGF-I receptor expression during refeeding suggests a role for the IGF action pathway in gut trophic responses to enteral nutrients. Other growth factor systems, such as the keratinocyte growth factor (KGF), stimulate, amongst others, the proliferation of intestinal epithelial cells(Reference Rubin, Osada and Finch85, Reference Housley, Morris and Boyle86), and are also regulated by the nutritional status of the animal(Reference Estívariz, Gu and Scully87). However, in contrast to intestinal IGF-I and IGF-I receptor mRNA(Reference Ziegler, Almahfouz and Pedrini5), intestinal KGF and KGF receptor mRNA levels were up-regulated during fasting, and returned towards control values after ad libitum refeeding. The increase in KGF and KGF receptor mRNA may be an adaptive response to maintain intestinal cell proliferation during starvation, when the expression and action of other growth factor systems, i.e. IGF-I, are blunted(Reference Estívariz, Gu and Scully87). Furthermore, it has been shown that the gene expression of several IGFBP changes in response to weaning. Different IGFBP show different patterns during postnatal development in the rat(Reference Shoubridge, Steeb and Read88) and also in the pig(Reference Tang, Van Kessel and Laarveld89). Such changes in the expression of the intestinal IGF system may be important for intestinal adaptive growth of the small-intestinal mucosa in response to weaning for protection against mucosal damage during, for example, inflammation(Reference Tang, Van Kessel and Laarveld89).
Table 2 Changes in intestinal gene expression due to fasting/refeeding in rats: effect on growth factors

IGF, insulin-like growth factor; ↓ , decrease; ↑ , increase; –, no effect; KGF, keratinocyte growth factor; GLP, glucagon-like peptide.
Glucagon-like peptides (GLP) represent the major secretory peptides derived from the post-translational processing of the proglucagon gene(Reference Brubaker and Drucker90, Reference Holst91). GLP-2 plays an essential role in maintaining the intestinal structure in the fed state, but also during adaptation in response to nutrient deprivation and subsequent feeding(Reference Shin, Estall and Izzo92). For young domestic animals, a potential therapeutic application of GLP-2 was suggested in periods of intestinal dysfunction and damage, such as during weaning and diarrhoeal diseases(Reference Burrin, Stoll and Guan93). Intestinal expression of proglucagon mRNA is influenced by the nutritional status of the animal. For example, in rats(Reference Hoyt, Lund and Winesett6) and mice(Reference Nian, Gu and Irwin94), fasting is associated with a decrease in intestinal proglucagon mRNA expression, while refeeding rapidly increases levels back to those of normal non-fasted controls (Table 2). Furthermore, in a study of Nelson et al. (Reference Nelson, Murali and Liu95) (Table 2), fasting in rats significantly decreased plasma concentrations of IGF-I and GLP-2, jejunal mucosal cellularity, IGF-I mRNA and ileal proglucagon mRNA. Upon intragastric and ad libitum refeeding, levels of plasma GLP-2 and jejunal IGF-I mRNA were elevated, accompanied by mucosal regrowth. This suggests that luminal nutrients stimulate intestinal growth, partly by an increased expression of both GLP-2 and IGF-I(Reference Nelson, Murali and Liu95). Furthermore, Petersen et al. (Reference Petersen, Hartmann and Holst96) found that enteral food supply decreased small-intestinal GLP-2 receptor mRNA abundance (which was highest at birth) in suckling and weaned pigs. The authors suggest that introduction of enteral feeding transiently increases plasma GLP-2 concentrations and decreases the levels of small-intestinal GLP-2 receptor mRNA during pig development. This emphasises the role of GLP-2 in the growth of the small intestine around birth and weaning due to a response to enteral nutrition.
Influence of dietary components on intestinal gene expression
Diet is a potent stimulant for altering the environment of cells in most organs, particularly in the GIT. A normal diet mainly consists of different proportions of carbohydrates, proteins and lipids, which may, along with vitamins and minerals, exert individual effects on intestinal gene expression(Reference Corthésy-Theulaz, Den Dunnen and Ferre47). In the following, examples are given where the major dietary constituents, i.e. carbohydrates, lipids and proteins/amino acids have been shown to affect intestinal gene expression. Besides, nutrients may escape digestion by mammalian enzymes in the small intestine. When present in the large-intestinal lumen, this material undergoes intense degradation by the colonic microflora resulting in the release of numerous bacterial metabolites. Fermentation of, for example, carbohydrates results, amongst others, in the production of SCFA(Reference Macfarlane, Cummings, Phillips, Pemberton and Shorter97). With regard to SCFA, especially butyrate has been shown to have multiple effects on cultured mammalian cells that include inhibition of proliferation, induction of differentiation and induction or repression of gene expression(Reference Davie18). However, other microbial metabolites also are able to influence intestinal gene expression. For example, polyamines, which originate, amongst others, from microbial proteolysis(Reference Macfarlane, Cummings, Phillips, Pemberton and Shorter97), but also from dietary sources, have been shown to modulate the expression of various genes involved in, for example, cell proliferation(Reference Li, Rao and Guo98, Reference Liu, Li and Rao99) or apoptosis(Reference Zhang, Rao and Guo100). Polyamines are required for the stimulation of mucosal growth, in part, through their ability to regulate the expression of nuclear transcription factors(Reference Liu, Li and Rao99, Reference McCormack and Johnson101, Reference Wang and Polyamines102). Thus, in the following, reference will also be made to studies focusing on intestinal gene expression as mediated by bacterial metabolites.
Protein and amino acids
Digestive function and nutrient metabolism
Typically, specific dietary components can affect the activity and gene expression of digestive enzymes and transporters. For example, the expression of the oligopeptide transporter Pept1 varies according to diet composition. In rats, a high-protein diet (50 %) resulted in a two-fold increase of Pept1 mRNA levels as compared with animals fed a low-protein diet (4 %)(Reference Erickson, Gum and Lindstrom103) or a protein-free diet(Reference Shiraga, Miyamoto and Tanaka104). In the study of Shiraga et al. (Reference Shiraga, Miyamoto and Tanaka104), rats were fed a diet supplemented with a single dipeptide (Gly-Phe) and phenylalanine for 3 d. The dipeptide and the amino acid enhanced transport activity, which was accompanied by an increase in Pept1 mRNA and protein in intestinal mucosal cells. Also, treatment of human intestinal cells with the dipeptide glycyl–sarcosine resulted in an increased Pept1 mRNA abundance, enhanced transport rate of the dipeptide glycyl–glutamine(Reference Thamotharan, Bawani and Zhou105) and vice versa(Reference Walker, Thwaites and Simmons106). In conclusion, endproducts of protein digestion, for example, small peptides, seem to participate in pathways that control the expression of intestinal transporters such as Pept1. This may lead to increased transporter capacity in the digestive tract(Reference Chen, Pan and Wong107), and results in an increased peptide transport, a characteristic of intestinal adaptation to a high-protein diet(Reference Adibi43, Reference Ferraris, Diamond and Kwan108). Although a practical use of peptides in livestock nutrition is still lacking, results of these studies suggest better nutritional management. Providing a diet that best accommodates the profile of digestive enzymes and nutrient transporters in the gut will result in improved nutrient utilisation of dietary protein, reduced N excretion, improved health and growth of the animal(Reference Gilbert, Wong and Webb39).
Furthermore, the intestinal peptide transport system is important for animals during the suckling period(Reference Xiao, Wong and Webb109). For example, Pept1 mRNA levels in the small intestine of rats increased rapidly from the early days to the middle of the suckling period, and then decreased from the middle to the end of the suckling period(Reference Shen, Smith and Brosius110). According to a study of Wang et al. (Reference Wang, Shi and Zhang111), Pept1 mRNA expression in the duodenum, proximal and distal jejunum of Tibetan piglets gradually increased from day 1 to the middle of the suckling period, and then gradually decreased from the middle to the end of the suckling period. The authors concluded that Pept1 gene expression was up-regulated by the onset of suckling colostrum or milk, which is rich in nutrients and growth factors, and thus the transport activity of the entire small intestine increased due to a dramatic increase in intestinal mucosal mass. However, from the middle to the end of the suckling period, the nutrients ingested by the piglets were limited by the milk yield of the sow, resulting in a decreased transport activity of the entire small intestine. Obviously, Pept1 mRNA expression along the small intestine is regulated according to age in early development, which may have major implications for amino acid and protein nutrition in young animals.
Dietary glutamine supplementation improves, amongst others, nutrient digestion and nutrient utilisation in early-weaned piglets(Reference Wu, Meier and Knabe112). Wang et al. (Reference Wang, Chen and Li7) showed, in response to dietary glutamine supplementation to piglets weaned on d 21, effects on small-intestinal gene expression that would provide an idea for the beneficial effects of glutamine. By using microarray technology, Wang et al. (Reference Wang, Chen and Li7) found, for example, an increased expression of genes related to lipid metabolism, Fe absorption and regulation of nutrient metabolism. For example, an increased expression was found for endozepine, which is expressed in intestinal mucosal cells, and which, amongst other functions, acts like acyl CoA-binding protein, thus regulating lipid metabolism, assembly and trafficking across the small intestine(Reference Steyaert, Tonon and Tong113, Reference Pusch, Jähner and Spiess114). Furthermore, an enhanced gene expression was observed for IL-13R-α-1, which is part of a receptor binding IL-13. The cytokine IL-13 stimulates contractility of intestinal smooth muscle, thereby promoting gut motility(Reference Morimoto, Morimoto and Zhao115). This may support the movement of luminal digesta along the small intestine, thereby facilitating the digestion of macronutrients by digestive enzymes and absorption of the resulting smaller molecules by enterocytes. However, on the other hand, an increase in intestinal motility also may cause excessive fluid losses, due to an increased transit time(Reference O'Loughlin, Scott and Gall116), which would then rather be a negative effect.
Immune function
In the study of Wang et al. (Reference Wang, Chen and Li7) with piglets weaned on day 21, the small-intestinal expression of several genes was down-regulated in response to dietary glutamine, including genes related to immune activation. However, there was an increased expression of genes related to defence against infection, such as IL-13R-α-1, and endozepine. Porcine endozepine has been shown to exert a potent antibacterial activity in the porcine gut(Reference Agerberth, Boman and Andersson117), while IL-13 is a multi-functional cytokine involved in host defence against infection, for example, against protozoans(Reference Zarlenga, Dawson and Kringel118). As early weaning is often associated with immunological challenges in the intestine(Reference Lallès, Bosi and Smidt119), elevated expression of, for example, endozepine in glutamine-supplemented diets for piglets may thus protect the gut from infections during weaning. Altogether, this study(Reference Wang, Chen and Li7) provides evidence for a possible molecular mechanism which would explain previous findings according to which dietary glutamine supplementation prevents intestinal dysfunction and enhances growth performance of early-weaned piglets(Reference Wu, Meier and Knabe112).
Arginine is a non-essential amino acid that has attracted interest because it plays an important role in many physiological and biological processes including physiology of the GIT(Reference Anggard120). It has been shown to be effective in a number of gut injury models(Reference Sukhotnik, Helou and Mogilner121, Reference Fu, Zhang and Zhang122). In a study of Liu et al. (Reference Liu, Huang and Hou123), dietary arginine supplementation alleviated intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide (LPS) in weaned pigs. In this study, 0·5 % arginine prevented the elevation of jejunal and ileal TNF-α mRNA abundance, and 1·0 % arginine alleviated the elevation of jejunal IL-6 mRNA and TNF-α mRNA abundance induced by LPS challenge. There was also a tendency for increased PPAR-γ mRNA abundance in the duodenum, jejunum (P < 0·05) and ileum due to arginine supplementation. These results indicate that arginine supplementation has beneficial effects in alleviating gut mucosal injury induced by LPS challenge, possibly by decreasing the expression of intestinal pro-inflammatory cytokines through activating PPAR-γ expression. PPAR-γ is a transcription factor and has been recognised as an endogenous regulator of intestinal inflammation(Reference Nakajima, Wada and Miki124). PPAR-γ ligands have been shown to be effective in intestinal inflammatory models(Reference Sato, Kozar and Zou125). The protective effects of PPAR-γ and its ligands are associated with the inhibition of inflammatory indices such as pro-inflammatory cytokines(Reference Moraes, Piqueras and Bishop-Bailey126). The inhibitory effect of PPAR-γ on pro-inflammatory cytokines could be mediated through the inhibition of transcription factor NF-κB(Reference Calkins, Bensard and Heimbach127, Reference Desreumaux, Dubuquoy and Nutten128).
Lipids
Digestive function and nutrient metabolism
Lipids of dietary origin can be involved in the regulation of expression of genes related to digestion and nutrient transport. For example, in a study of Yasutake et al. (Reference Yasutake, Goda and Takase129), 6-week-old rats were fed either a diet high in medium-chain TAG (MCT), a diet high in long-chain TAG or a high-carbohydrate diet. As compared with the long-chain TAG diet, the MCT diet as well as the carbohydrate diet resulted in jejunal elevation of mRNA of sucrase–isomaltase, and of Na-coupled glucose co-transporter-1 (SGLT1), by which glucose enters the epithelial cells via active transport(Reference Hediger, Coady and Ikeda130). Force-feeding the MCT or a high-sucrose diet evoked a marked increase in sucrase–isomaltase mRNA and SGLT1 mRNA levels within 12 h, as compared with force-feeding a long-chain TAG diet. This suggests that not only carbohydrate intake but also MCT intake might influence jejunal sucrase–isomaltase mRNA and SGLT1 mRNA levels, probably through a generation of common metabolite(s) of carbohydrates and MCT(Reference Yasutake, Goda and Takase129). According to the study of Yasutake et al. (Reference Yasutake, Goda and Takase129), glycerol as such a metabolite originating from MCT might enter the glycolytic pathway and mimic the effect of a high-carbohydrate diet.
Furthermore, the high lipid content of mother's milk might play a role in intestinal gene expression of newborn animals. For example, in neonatal piglets given a high-TAG intraduodenal infusion for 24 h, jejunal apoA-IV expression increases 7-fold at the pretranslational level in comparison with control animals given a low-TAG infusion(Reference Black, Rohwer-Nutter and Davidson131, Reference Black, Wang and Hunter132). ApoA-IV is expressed in the small intestine and plays a role in lipid absorption, transport and metabolism(Reference Stan, Delvin and Lambert133). In the older, suckling piglet, only a two-fold increase could be shown. This might be of importance in the newborn receiving a high-fat milk diet, when apoA-IV may play a role in facilitating enterocyte lipid transport under such conditions of high lipid flux(Reference Lu, Yao and Meng134). More recently, it has been shown in developing rats(Reference Mochizuki, Mochizuki and Kawai135) that fatty acids in mother's milk induce gene expression of the liver-type fatty acid-binding protein (L-FABP) and cellular retinol-binding protein type II (CRBPII), which are cytosolic binding proteins for fatty acids and vitamin A, facilitating their absorption from lumen to the enterocytes. In this study, jejunal mRNA levels of PPAR-α were increased during the suckling period. Furthermore, the authors examined the expression of these genes following oral administration of a range of fatty acids, namely caprylic acid, oleic acid, linoleic acid and arachidonic acid, all of which are components of the milk of weaning rats. PPAR-α, L-FABP and CRBPII were induced by caprylic and oleic acids to similar extents. The results of this study indicate that the expression of small-intestinal L-FABP and CRBPII genes in postnatal development is regulated by a change in expression of PPAR-α which transmits the signalling of fatty acids derived from milk(Reference Mochizuki, Mochizuki and Kawai135). For the young animal, this suggests that fatty acids in milk may help to ensure the effective absorption of dietary fat and vitamin A.
Immune function
Several studies have been performed investigating the effects of dietary lipids on intestinal gene expression with regard to immunological parameters, mostly directed to intestinal inflammation. According to a study with rats(Reference Kono, Fujii and Asakawa136), MCT enhanced secretory IgA (sIgA) expression in the ileum compared with rats given maize oil. After LPS challenge, expression of sIgA was decreased in the maize oil group but not in the MCT group. Furthermore, MCT administration resulted in a decreased expression of pro-inflammatory cytokines (TNF-α and IL-18), and an increased expression of IgA-promoting cytokines (IL-6, IL-10), together with lower mRNA expression of the IgA-inhibiting cytokine IFN-γ. Since sIgA plays an important role in protection against infections in the intestinal immune system, an enhanced sIgA production is thought to be beneficial for the organism(Reference Mayer137). Obviously, dietary MCT can reduce intestinal injury after endotoxin administration by inhibition of the expression of some inflammatory cytokines and enhancing intestinal sIgA(Reference Kono, Fujii and Asakawa136).
Furthermore, effects on intestinal gene expression have been shown in several studies investigating the anti-inflammatory effects of dietary conjugated linoleic acid (CLA) (Table 3). Hontecillas et al. (Reference Hontecillas, Wannemeulher and Zimmerman138) could show in pigs with induced colitis that supplementation with CLA before the induction of colitis decreased mucosal damage and maintained cytokine expression and lymphocyte subset distribution similar to that in non-infected pigs. Furthermore, CLA supplementation led to an enhanced colonic expression of PPAR-γ. Accordingly, Bassaganya-Riera et al. (Reference Bassaganya-Riera, Reynolds and Martino-Catt139) found amelioration of colitis in mice due to CLA-supplemented diets, mediated through a PPAR-γ-dependent mechanism. PPAR-γ belongs to a group of transcription factors that are sensitive to dietary fatty acids(Reference Kersten, Desvergne and Wahli12), and thus may translate nutritional stimuli into changes in gene expression(Reference Schoonjans, Staels and Auwerx11). Activators of PPAR-γ have been shown to inhibit the activation of a number of inflammatory genes(Reference Jiang, Ting and Seed13, Reference Marx, Sukhova and Collins14). The anti-inflammatory actions of PPAR may occur via stimulation of the breakdown of inflammatory eicosanoids through the induction of peroxisomal β-oxidation(Reference Delerive, Fruchart and Staels140, Reference Levy, Clish and Schmidt141). However, they may also interfere with or antagonise the activation of other transcription factors, including NF-κB(Reference Ricote, Li and Willson142), thereby suppressing the expression of pro-inflammatory cytokines (i.e. TNF-α, IL-6 and IL-1β)(Reference Desreumaux, Dubuquoy and Nutten128). In a further study of Bassaganya-Riera & Hontecillas(Reference Bassaganya-Riera and Hontecillas143), using a pig model with induced colitis, dietary CLA supplementation up-regulated colonic PPAR-γ expression and contributed to a delay in the onset of induced colitis and an attenuated growth depression in the pigs, coupled with down-regulation of TNF-α. Similarly, Changhua et al. (Reference Changhua, Jindong and Defa144) found that dietary CLA attenuated the production and expression of pro-inflammatory cytokines in the spleen and thymus of weaned pigs challenged with LPS, mediated at least in part through a PPAR-γ-dependent mechanism, together with an enhanced production and expression of the anti-inflammatory cytokine IL-10. These studies suggest the potential of dietary CLA in alleviating mucosal damage originating from intestinal inflammation, thereby possibly improving overall gut health.
Table 3 Effect of dietary conjugated linoleic acid supplementation on gene expression related to immune function in pigs
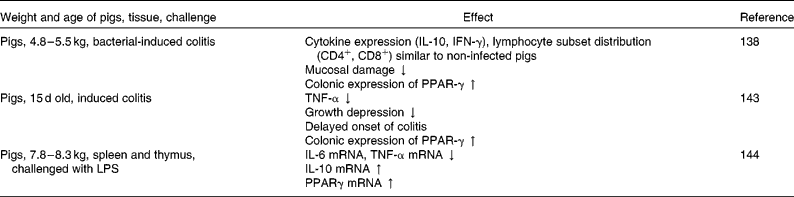
IFN, interferon; ↓ , decrease; ↑ , increase; LPS, lipopolysaccharides.
Carbohydrates
Digestive function and nutrient metabolism
Many studies have shown that feeding a high-carbohydrate diet induces the intestinal expression of genes related to carbohydrate digestion and absorption, including disaccharidases and monosaccharide transporters(Reference Tanaka, Kishi and Igawa145, Reference Kishi, Tanaka and Igawa146). For example, upon the introduction of a high-starch diet to rats, both sucrase and lactase activities were elevated, accompanied by the accumulation of the respective mRNA(Reference Yasutake, Goda and Takase129, Reference Goda, Yasutake and Suzuki147). Also, expression of the SGLT1 gene is up-regulated by feeding rodents a carbohydrate-rich diet(Reference Yasutake, Goda and Takase129, Reference Kishi, Tanaka and Igawa146). Furthermore, dietary fructose (or a metabolite of it) leads to an increase in the transcripts of sucrase–isomaltase and lactase genes in rats, as well as for the transcripts of the transporter (GLUT5) genes(Reference Tanaka, Kishi and Igawa145, Reference Kishi, Tanaka and Igawa146). Thus, transcriptional regulation is involved in the dietary adaptation of the carbohydrate digestion/absorption-related genes, and regulation of enzymes and transporters seems to be linked to each other(Reference Goda148). However, according to a study of Cui et al. (Reference Cui, Soteropoulos and Tolias149), using microarray hybridisation and relative quantitative reverse transcription-PCR, a wide range of genes changed in their expression in response to a fructose perfusion of the rat small intestine. Along with expression of the intestinal brush-border fructose transporter GLUT5, expression of more than twenty genes increased considerably, including two gluconeogenic enzymes, glucose-6-phosphatase and fructose-1,6-bisphosphatase, and fructose-2,6-bisphosphatase, an enzyme unique to fructose metabolism and regulating fructose-1,6-bisphosphatase activity. Furthermore, not only genes related to sugar metabolism and transport, but also other genes changed their expression in response to high fructose perfusion, for example, transcription factors. This shows that many signalling and metabolic pathways in the small intestine are responsive to the presence of one single nutrient, i.e. in this case fructose, in the intestinal lumen. In addition, it has been shown in rats that the intestine is fructose insensitive before approximately age 14 d, i.e. GLUT5 can be regulated by fructose when pups are weaning but not when pups are suckling(Reference Jiang and David150). Thus, the ontogenic development of the intestine determines when substrate regulation of GLUT5 can occur. GLUT5 is a model of developing transporter systems in the small intestine, as it has sharply defined stages that are characterised by differences in the ability of its substrate to regulate GLUT5 transcription. Furthermore, it was shown that a premature induction of GLUT5 expression and fructose uptake can be induced by glucocorticoids in suckling rats aged 10 d(Reference Douard, Cui and Soteropoulos151). Biologically, this could mean that, in case the mother is under stress, it may release high levels of glucocorticoids with the milk(Reference Lesage, Blondeau and Grino152) that subsequently and prematurely enable the small intestine of the young to digest and absorb nutrients from the environment, thereby improving its survival rate in the event of the loss of the mother. This indicates developmental plasticity providing the ability of the intestine to change function in response to environmental cues(Reference Douard, Cui and Soteropoulos151). Although these studies have been performed with rats, they provide valuable information for dietary regulation of nutrient digestion and transport by their respective substrates, but also that specific nutrients are able to influence intestinal gene expression beyond their own metabolism. Furthermore, with regard to GLUT5 regulation, it becomes clear that several factors may be involved in regulatory mechanisms, i.e. substrate and age, but that there also exists the possibility to modify regulatory mechanisms by external factors, for example, by diet.
Fermentable carbohydrates
Digestive function and nutrient metabolism
Tako et al. (Reference Tako, Glahn and Welch153) assessed the effect of dietary inulin (4 %) on the gene expression of selected intestinal Fe transport proteins in anaemic piglets (aged 5 weeks, injected with only half of the normal Fe dose, 50 mg Fe as Fe-dextran), using semi-quantitative RT-PCR analyses (Table 4). In this study, mRNA levels of intestinal Fe transporters, enzymes and binding proteins in duodenal samples were significantly higher in the inulin group compared with the control. In the colon, expression of the pig divalent metal transporter 1, transferrin receptor and ferritin significantly increased for the inulin group as well. Furthermore, a 100 % increase in duodenal mucin mRNA level was observed for the inulin group. For fermentable carbohydrates such as inulin, it can be assumed that they may undergo microbial fermentation in the large intestine leading to the production of SCFA(Reference Williams, Verstegen and Tamminga19, Reference Macfarlane, Cummings, Phillips, Pemberton and Shorter97), although considerable amounts may already be fermented before the distal ileum(Reference Loh, Eberhard and Brunner154, Reference Mikkelsen and Jensen155). Although SCFA concentrations were not determined in the study of Tako et al. (Reference Tako, Glahn and Welch153), there were significant increases in caecal populations of lactobacilli and bifidobacteria, suggesting also a possible effect on SCFA concentrations. Thus, according to the authors, one possible pathway by which inulin might affect intestinal gene expression would be via an increased SCFA production in the colon leading to a possible systemic effect of butyrate on Fe metabolism.
Table 4 Effect of dietary supplementation with fermentable carbohydrates on intestinal gene expression in piglets

↑ , Increase; TGF, transforming growth factor; IGF, insulin-like growth factor; ↓ , decrease.
Immune function
An example for the modification of inflammatory conditions by dietary means is provided by a study of Kanauchi et al. (Reference Kanauchi, Serizawa and Araki156) in mice with induced colitis that were fed a diet containing germinated barley (defined as a prebiotic product rich in hemicelluloses, which also contains glutamine-rich protein). This dietary treatment prevented disease activity after induction of colitis, associated with increased caecal butyrate concentration, decreased serum IL-6 levels as well as a tendency for reduced NF-κB expression. The authors suggest that the anti-inflammatory effects of the germinated barley may be due to increased butyrate production, possibly mediated via inhibition of NF-κB.
In Table 4, studies pertaining to effects of dietary supplementation with fermentable carbohydrates on intestinal gene expression in pigs are summarised. For example, Pié et al. (Reference Pié, Awati and Vida74) investigated the effect of added fermentable carbohydrates on the mRNA content of several pro-inflammatory cytokines (for example, IL-1β, IL-6, IL-8, IL-18, TNF-α) in the ileum and colon of newly weaned piglets. Fermentation endproducts were also assessed. The carbohydrate-enriched diet induced an up-regulation of IL-6 mRNA content in the colon of piglets 4 d post-weaning, which was not observed for the piglets fed the control diet. This indicates that the regulation of IL-6 mRNA in the colon depends on dietary factors, or at least on microbiological factors that have been influenced by the diet. However, in this study, comparisons of IL-6 mRNA contents with SCFA (acetic, propionic and butyric acids) concentrations in the ileum and colon showed no correlations, indicating that the increased level of this cytokine was not directly dependent of the presence of SCFA. In addition, an increase in IL-1β mRNA was observed at day 4 post-weaning in piglets fed the carbohydrate diet, and also in the control group, suggesting that the level of this cytokine is not influenced by diet composition. In another study with piglets(Reference Pié, Lallès and Blazy27), a rapid increase in IL-1β after weaning was obtained. Obviously, the observed responses are more general, i.e. reflecting early immunological changes associated with weaning, or other stress, in piglets. A further study with piglets (aged 15 d) fed fermentable carbohydrates showed enhanced IL-1β mRNA levels in the jejunal mucosa and mesenteric lymph nodes, as well as increased serum levels of IL-1β(Reference Yin, Tang and Sun157). Consistently, serum levels of IL-2 and IL-6 in piglets fed the carbohydrates were higher than in control animals. The authors suggested that such an increase in inflammatory cytokines may result from activation of some intestinal bacteria which then stimulated an immune defence response(Reference Yang, Wang and Li158). Schedle et al. (Reference Schedle, Pfaffl and Plitzner159) used a piglet model to investigate the immunological response of gastrointestinal tissues to dietary additions of insoluble fibre, either wheat bran (rich in cellulose and hemicelluloses) or pine pollen (rich in lignin), measured as relative mRNA expression levels of different transcription factors (NF-κB), inflammatory marker genes (TNF-α, TGF-β), as well as markers of the cell cycle (caspase 3, CDK4) and growth factors (Table 4). According to this study, NF-κB, TNF-α, TGF-β and caspase 3 were up-regulated in the proximal part of the GIT due to supplemental wheat bran, suggesting that the impact of insoluble dietary fibre is not limited to the colon. Furthermore, due to a down-regulation of NF-κB in the colon caused by pine pollen, the authors suggested a reduction in pro-inflammatory action in colonic tissue. In this study, Schedle et al. (Reference Schedle, Pfaffl and Plitzner159) also measured morphological parameters, and found an increased villous height of the mucosa in the jejunum and ileum due to insoluble dietary fibre. In addition, TGF-β was up-regulated in the stomach and jejunum by insoluble fibre, which is in line with a pronounced cell proliferation and an increase in villus size. They concluded that additions of insoluble fibre rich in lignin might significantly contribute to GIT stability, in terms of increased cell proliferation and reduced inflammation in weaning piglets, however, not only with regard to the colon, but rather to the entire digestive tract. However, the insoluble fibre used in this study appeared to be not fermentable. So, no relationships to bacterial metabolites were drawn and the primary mode of action remains unclear.
Growth factors
Several studies in, for example, rats and dogs(Reference Massimino, McBurney and Field160, Reference Cani, Dewever and Delzenne161) have shown that diets supplemented with fermentable carbohydrates up-regulate the levels of intestinal proglucagon mRNA, as well as the amount of proglucagon-derived peptides (PGDP), when compared with diets containing no or a low content of fermentable carbohydrates (Table 5). The increase in intestinal proglucagon expression is probably due to carbohydrate fermentation in the colon and its simultaneous production of SCFA(Reference Reimer and McBurney162–Reference Tappenden, Thomson and Wild164). Indeed, SCFA are known to increase proglucagon mRNA in the ileum of rats(Reference Tappenden, Drozdowski and Thomson165, Reference Drozdowski, Dixon and McBurney166). With regard to the various physiological effects of intestinal PGDP, for example, intestinotrophic function, a stimulation of intestinal PGDP synthesis and secretion by ingestion of specific dietary components might be of interest during disease or intestinal insufficiency. For GLP-1, but also for GLP-2, it seems that there is the possibility to modulate its endogenous availability via an enhanced production of the precursor proglucagon mRNA through the ingestion of fermentable carbohydrates(Reference Thulesen, Hartmann and Nielsen167–Reference Delzenne, Cani and Daubioul170).
Table 5 Effect of fermentable carbohydrates on intestinal proglucagon gene expression and proglucagon-derived peptide production

↑ , Increase; GLP, glucagon-like peptide.
Bacterial community and metabolites
Microbiota–host interactions
Extensive cross-talk between the microbiota and the intestinal epithelium takes place, and the intestinal microbiota influences the GIT and the host as such(Reference Leser and Mølbak171). Thus, modulation of the bacterial community by dietary ingredients, such as carbohydrate sources(Reference Castillo, Skene and Roca172), can have sustainable effects on intestinal development and integrity. For instance, activities of brush-border enzymes, such as disaccharide hydrolases, peptidases and alkaline phosphatase, which are markers of enterocyte differentiation, are modified by intestinal bacterial colonisation(Reference Kozakova, Kolinska and Lojda173, Reference Siggers, Shirkey and Drew174). In gnotobiotic animals, i.e. germ-free animals colonised with one or a few bacterial species, the effects of individual bacterial strains or defined groups of strains on the host epithelium can be investigated. For instance, Hooper et al. (Reference Hooper, Wong and Thelin175) used a gnotobiotic mouse model to examine gene expression responses following monoassociation with Bacteroides thetaiotaomicron. Using DNA microarrays, laser-capture microdissection and quantitative RT-PCR, Hooper et al. (Reference Hooper, Wong and Thelin175) determined that B. thetaiotaomicron modulated the expression of seventy-one intestinal genes involved in nutrient absorption, mucosal barrier fortification, xenobiotic metabolism, angiogenesis and postnatal intestinal maturation. Colonisation of conventional mice with Lactobacillus paracasei spp. paracasei F19 or L. acidophilus NCFB 1748 induced significant regulation of twenty-two and fifty-five genes involved in essential physiological functions such as the immune response, regulation of energy homeostasis and host defence in the distal ileum, respectively, whereas only twenty genes were regulated by both strains(Reference Nerstedt, Nilsson and Ohlson176). Using a neonatal pig model, Siggers et al. (Reference Siggers, Shirkey and Drew174) showed that monoassociation of pigs with L. fermentum stimulated the prohormone proglucagon expression, whereas monoassociation with E. coli decreased it. Many cleaved peptides from proglucagon have important biological activities, such as GLP-2 that is involved in epithelial proliferation, decreased epithelial apoptosis and increased nutrient absorption(Reference Burrin, Stoll and Jiang177). Moreover, Willing & Van Kessel(Reference Willing and Van Kessel178) demonstrated that association of neonate piglets with conventional bacteria or monoassociation with E. coli but not with L. fermentum increased overall cell turnover in the small intestine by stimulating increased apoptosis through the expression of death ligand Fas ligand and TNF-α and by increasing cell proliferation. Throughout the GIT the most significant molecular function affected by the microbiota is the activity of proteins involved in defence or immune responses(Reference Leser and Mølbak171), and all genes with this annotation are up-regulated in the small and large intestines of conventional mice and piglets compared with germ-free animals(Reference Mutch, Simmering and Donnicola179, Reference Shirkey, Siggers and Goldade180). When germ-free piglets were conventionalised, epithelial inflammatory responses were detected in the ileum using high-density porcine oligonucleotide microarrays. Genes involved in epithelial cell turnover, mucus biosynthesis and priming of the immune system were induced. Receptors and transcription factors related to IFN-inducible genes were up-regulated; however, at the same time, the inflammatory response seemed to be controlled through activation of genes in pathways that prevent excessive inflammation, supporting the concept of a homeostatic epithelium that maintains a tight intestinal barrier without producing excessive inflammatory responses, which would compromise barrier function(Reference Chowdhury, King and Willing181). Overall, the microbiota affects a multitude of epithelial functions in the intestine as a consequence of specific bacterial–host interactions, and these extend far beyond the immune response (for a review, see Leser & Mølbak(Reference Leser and Mølbak171)).
SCFA
The ability of SCFA, particularly butyrate, to influence gene expression is increasingly considered. One well-known mechanism of butyrate to influence gene expression is via the induction of histone hyperacetylation through the inhibition of histone deacetylase (HDAC). By this means, the compactness of the histone is reduced, and the DNA is more accessible to transcription factors(Reference Kruh182, Reference McCaffrey, Newsome and Fibach183). Among the different SCFA with different chain lengths, butyrate is most effective in inhibiting HDAC activity(Reference Kruh182, Reference Fusunyan, Quinn and Fujimoto184). In Table 6, in vitro studies using cultured intestinal cells to investigate the influence of butyrate on cytokine gene expression are summarised. This might be of special importance with regard to inflammatory diseases, as particularly butyrate has been shown to suppress intestinal inflammation (for example, Harig et al. (Reference Harig, Soergel and Komorowski185)). For example, colonic secretion of IL-8, a chemotactic and activating peptide for neutrophils(Reference Izzo, Witkon and Chen186, Reference MacDermott, Sanderson and Reinecker187), is increased in human ulcerative colitis ex vivo, but is suppressed by butyrate(Reference Gibson and Rosella188). A possible molecular mechanism to mediate the effects of butyrate is via modification of transcription factor activation. Segain et al. (Reference Segain, Raingeard de la Bletiere and Bourreille189) showed in LPS-stimulated lamina propria mononuclear cells, and in the colonic mucosa of mice with induced colitis that butyrate prevented the nuclear translocation of NF-κB. The inhibitory effect of butyrate on NF-κB activation was also confirmed by Zapolska-Downar et al. (Reference Zapolska-Downar, Siennicka and Kaczmarczyk190) in endothelial cells. It seems that the anti-inflammatory effects of butyrate – at least in part – are mediated via inhibition of NF-κB(Reference Segain, Raingeard de la Bletiere and Bourreille189) which regulates the expression of genes involved in the inflammatory response, for example, cytokines such as TNF(Reference Baldwin191). It has been proposed that the inhibitory effect of butyrate on NF-κB activation originates from its ability to inhibit HDAC activity(Reference Inan, Rasoulpour and Yin192). On the other hand, Weber & Kerr(Reference Weber and Kerr193) could not find in vivo effects of dietary sodium butyrate on the ileal relative abundance of cytokine mRNA (IL-6, TNF-α) in weanling pigs challenged with E. coli LPS. This is in contrast to Milo et al. (Reference Milo, Reardon and Tappenden194) who found in young pigs that parenterally fed SCFA increased intestinal IL-6 abundance. Weber & Kerr(Reference Weber and Kerr193) hypothesised that due to the anti-inflammatory activity of butyrate(Reference Zhang, Yao and Lu195) and other HDAC inhibitors(Reference Leoni, Fossati and Lewis196), butyrate might decrease the cytokine response to LPS. The lack of response in the study of Weber & Kerr(Reference Weber and Kerr193) could be due to the different routes of administration for butyrate, or could be due to the fact that Milo et al. (Reference Milo, Reardon and Tappenden194) used a combination of SCFA.
Table 6 Influence of butyrate on cytokine gene expression of cultured intestinal cells

HIMEC, human intestinal microvascular endothelial cells; LPS, lipopolysaccharide; ↓ , decrease; –, no effect; ↑ , increase; MCP-1, monocyte chemoattractant protein-1.
With regard to effects on growth factors, it has been shown in vitro that butyrate, and to a lesser extent other SCFA, increased the synthesis of IGFBP-2 by the intestinal epithelial cell line Caco-2 (via histone acetylation). Simultaneously, synthesis of IGFBP-3, which is predominantly secreted in the absence of SCFA, is decreased(Reference Nishimura, Fujimoto and Oguchi197). Changes in IGFBP secretion corresponded to mRNA accumulation. Altering the profile of IGFBP may have profound effects on the bioavailability of IGF-I and IGF-II. Obviously, epithelial cells respond to bacterial products, i.e. SCFA, by secreting proteins that may modulate factors that affect their own proliferation and that of other intestinal cell types(Reference Nishimura, Fujimoto and Oguchi197). With regard to the proliferative effects of the IGF system, for example, on intestinal epithelial cells, it has been suggested that changes in the expression of IGF-I, IGF-IR and IGFBP in the intestine may play an important role in limiting mucosal damage or promoting tissue repair. This might be of importance for the adaptive function of the small intestine in response to weaning and during inflammation(Reference Tang, Van Kessel and Laarveld89, Reference Lund and Zimmermann198).
Polyamines
Expression of enzymes and transporters related to digestion and absorption may also be influenced by polyamines(Reference Wild, Searles and Koski199), originating from microbial proteolysis(Reference Macfarlane, Cummings, Phillips, Pemberton and Shorter97), or from dietary sources(Reference Blachier, Mariotti and Huneau200), for example, from mother's milk(Reference Motyl, Ploszaj and Wojtasik201). In a study of Wild et al. (Reference Wild, Searles and Koski199), it has been shown that oral polyamine administration may modify the ontogeny of hexose transporter gene expression in the postnatal rat intestine. In this study, the polyamine spermidine, supplied at a level similar to the concentration in rat breast milk(Reference Schultz, Hudson, Field and Frizzell202), increased the abundance of mRNA for sucrase–isomaltase, SGLT1 and GLUT2, as well as ornithine decarboxylase (ODC) activity and protein and mRNA abundance. The maturational changes in enzyme expression have been correlated to both mucosal polyamine levels and to the onset of the expression and activity of ODC, the key enzyme of polyamine biosynthesis(Reference Luk, Marton and Baylin203). The study of Wild et al. (Reference Wild, Searles and Koski199) suggests that normal ontogenic expression of the digestion and absorption of glucose may be modified by an exogenous polyamine (spermidine), acting through the increase in ODC activity, which, in turn, enhances the mRNA and protein abundance of the enzymes and transporters involved in this process.
Furthermore, polyamines originating from microbial proteolysis may be involved in the expression of genes related to the innate immune system. Chen et al. (Reference Chen, Rao and Zou204) showed in vitro, by use of a cell line from the normal rat small intestine (IEC-6 cell line), that polyamines are necessary for Toll-like receptor (TLR)2 expression. Intestinal epithelial cells (IEC) express a variety of potential sensing receptors, including TLR, which are fundamental components of the innate immune response and allow mammalian intestinal epithelium to detect various microbes and different pathogens(Reference Abreu, Fukata and Arditi205, Reference Janeway and Medzhitov206). Thus, the polyamine-modulated TLR2 activation plays an important role in the regulation of the epithelial barrier function. It seems that polyamines are crucial for the maintenance of the epithelial barrier integrity and mucosal homeostasis under physiological conditions(Reference Chen, Rao and Zou204).
Limitations and strengths of gene expression to determine implications of nutrition during weaning
Research has focused on mechanisms controlling gene expression, and the impact of biological and external factors on gene expression. Before the genomic approach was introduced in science, molecular biology aimed at investigating single genes or proteins, and their structure and function, in isolation from the larger context of other genes. The limitation of this approach is the need for a working hypothesis. Furthermore, it appears that physiological processes are governed by several genes acting in concert rather than by only one or a few individual genes. Genomic technologies such as array technology and large-scale DNA sequencing techniques facilitated the analysis of thousands of genes or proteins or metabolites in a single experiment (genomic approach). This approach is generating hypotheses and allows the identification of novel relevant genes to be further studied(Reference Cassar-Malek, Picard and Bernard207).
However, it is of major importance to identify interdependences between different classes of measurements (for example, zootechnical data v. histological v. gene expression data)(Reference Schedle, Pfaffl and Plitzner159), and to obtain more additional information on possible modes of action by methods of bioinformatics (cluster analysis, comparison of thousands of expressed mRNA)(Reference D'Haeseleer208). For example, Hamard et al. (Reference Hamard, Mazurais and Boudry209) investigated both the physiological disturbances and molecular events caused by an inadequate threonine supply to the ileum of piglets. Gene expression profiles were compared with responses of physiological origin. Most of the changes were consistent with an increase in metabolic activity including, for example, protein synthesis. The authors concluded that comparison of transcriptomic and physiological data may allow a better understanding of the functional implication of these genes as well as associated mechanism of regulation. However, to validate links between changes of gene expression and function, it is necessary to confirm biological interpretation of these results. Furthermore, it has to be kept in mind that data from gene expression studies may sometimes appear relatively unclear, because they are reflecting reactions of homogenised tissue segments, comprising different cell types. Thus, it would be necessary to link mRNA measurements with specific individual cell types (for example, by means of micro-dissection or immune histological techniques)(Reference Schedle, Pfaffl and Plitzner159).
In consequence, although a direct correlation between transcriptomic regulation and functional impact is not always easy to establish, studies exist suggesting fasting/refeeding-induced gene regulations (for example, Mazurais et al. (Reference Mazurais, Romé and Cahu35)) to be consistent with alterations in the small intestine in piglets at weaning (for example, Marion et al. (Reference Marion, Biernat and Thomas210); Boudry et al. (Reference Boudry, Peron and Le Huërou-Luron24)). Thus, although given the limitation of gene expression studies, for example, need for comparisons between changes in gene expression with other, for example, morphological or zootechnical parameters, use of gene expression studies will help to gain deeper insight into the mechanism governing the physiological functions and their regulation in livestock species(Reference Cassar-Malek, Picard and Bernard207). In that way, it will become possible to evaluate feeding regimens, compounds for non-pharmaceutical prevention of infectious enteral disease, and evaluation of pharmaceutical treatment of enteral diseases(Reference Niewold, Kerstens and Van der Meulen36).
Implications for pig nutrition
Dietary invention to prevent disease through feed supplementation has become a standard procedure in modern pig production systems. This requires, however, understanding of the ability to manipulate a multitude of nutrient-related interactions at the gene, protein and metabolic levels. Comparison of transcriptomic and physiological data may allow a better understanding of the functional implication of these genes as well as associated mechanisms of regulation(Reference Hamard, Mazurais and Boudry209). Although direct correlation between transcriptomic regulation and functional impact is not always easy to establish, numerous studies have shown that nutrition-induced gene regulations observed are consistent with changes in intestinal structure and function.
By use of microarray technology, it has been shown that early weaning may lead to an increased expression of genes that promote oxidative stress and immune activation but decreased expression of genes related to nutrient utilisation and cell proliferation in the piglet's small intestine(Reference Wang, Chen and Li7). However, as weaning generally is associated with a reduced feed intake(Reference Bruininx, Binnendijk and van der Peet-Schwering21), a clear distinction between effects of weaning and associated reduced feed intake on gene expression seems to be impossible. Also, weaning at early maturation stages frequently causes mucosal inflammation, which can be seen from an enhanced expression of pro-inflammatory cytokines. Thus, nutritional strategies are in favour that are capable of manipulating the secretion of pro-inflammatory cytokines(Reference Changhua, Jindong and Defa144). Specific dietary fatty acids (for example, CLA) may have therapeutic potential, since they are able to influence the inflammatory response via specific transcription factors(Reference Bassaganya-Riera, Reynolds and Martino-Catt139). Furthermore, specific feed ingredients, such as linseed, have been suggested as potential functional ingredients which may affect intestinal gene expression in young piglets after weaning(Reference Jansman, Niewold and Hulst211). Linseed contains a number of constituents, such as mucilage, gel-forming polysaccharides, structural carbohydrates and n-3 fatty acids, which could have functional properties in relation to maintaining and supporting gastrointestinal health in post-weaning piglets. For example, mRNA abundance of a lectin with anti-inflammatory properties which is expressed in the intestinal epithelium and involved in innate defence mechanisms was higher in linseed-fed pigs than in pigs fed the control diet. Stimulation or inhibition of such genes by feeding piglets a diet with specific functional constituents may be a promising way to improve intestinal health under stressful conditions such as weaning.
Also, providing a diet that best accommodates the profile of digestive enzymes and nutrient transporters present in the gut will result in improved utilisation of dietary nutrients, improved health and growth. For example, from a commercial livestock industry perspective, dietary protein is a costly nutrient, and even fractional improvements in its utilisation have the potential to reduce production costs as well as reduce the N excretion into the environment. Although dietary protein regulation of peptide transporter expression and activity has not been intensively studied in livestock, much of the work conducted in species used in biomedical research is broadly applicable, and provides a starting point for other studies aimed at determining the nutritional relevance of peptide transport(Reference Gilbert, Wong and Webb39). Further approaches may be based on the stimulation and promotion of rapid gastrointestinal development, thus supporting the animal to better cope with dietary changes associated with weaning. In this context, brush-border enzymes, such as lactase or maltase, are frequently used as cell markers of villus maturation(Reference James, Smith and Tivey212–Reference Lifschitz, Smith and Garza214). Accordingly, supplementation with specific dietary ingredients might offer the possibility to enhance proliferation and differentiation of epithelial cells and stimulate enzyme production in the upper GIT of weaned piglets.
Effects of specific dietary components may also be mediated rather indirectly via the metabolites produced from these components by the gastrointestinal microbiota, such as SCFA from carbohydrate fermentation or polyamines, which originate from protein fermentation. By changing the source and quantity of components available as fermentable substrates in the distal parts of the GIT, it may be possible to alter the amount and composition of microbial metabolites. For the SCFA, and particularly butyrate, it has been shown that they can influence the expression of various genes in the intestine, including, for example, cytokine expression. According to Pié et al. (Reference Pié, Awati and Vida74), a diet enriched in fermentable carbohydrates can modulate the IL-6 mRNA content during the weaning period in piglets. Correlations between several pro-inflammatory cytokines and the endproducts of fermentation demonstrated that the regulation of cytokines is strongly linked to fermentation endproducts. As cytokines play a major role in the maintenance of gastrointestinal homeostasis, their regulation by nutritional factors may be an important consideration in the future for the development of new diet formulas for newly weaned piglets. Furthermore, dietary regulation of intestinal proglucagon expression via the ingestion of fermentable carbohydrates, associated with an increase in SCFA production, seems to be a promising approach in view of the physiological effects of intestinal PGDP. GLP-2, which may act as a physiological regulator of intestinal growth, has been suggested as a treatment for gastrointestinal diseases characterised by intestinal dysfunction. A stimulation of endogenous intestinal PGDP synthesis by the ingestion of specific dietary components could represent an alternative approach to exogenous GLP administration, and might be of considerable interest for therapeutic approaches.
However, it has to be kept in mind that changes in the intestine as they occur at weaning are often more a consequence of reduced feed intake, stress and inflammation than of dietary changes. This has been shown when investigating the impact of switching from milk-based to cereal-based diets on the morphology and function of the small intestine in piglets to isolate the influence of the diet from that of other factors, such as social or environmental(Reference Boudry, Lallès and Malbert215). With regard to mucosal integrity, the effect of diet composition seems not to be as important as the sequential effects of low feed intake during the first 4 d post-weaning. Accordingly, alterations of intestinal integrity were also observed in underfed piglets and piglets receiving TPN, when compared with their counterparts ad libitum or receiving enteral nutrition, independently of the weaning process(Reference Carey, Hayden and Tucker26, Reference Park, Monaco and Donovan56). Thus, based on the present review, one major consequence would be stimulation of feed intake of piglets in the early post-weaning phase through various measures. This includes the use of specific feed ingredients that are well accepted by the animals. For example, dairy products, such as whey or skimmed milk powder have favourable effects on feed intake, and thus on health and growth performance(Reference Thacker216). Or, providing a weaner diet in liquid form enhances feed intake and thus health(Reference Brooks, Moran, Beal, Varley and Wiseman217). Also, supplementation of weaner diets with animal protein, for example, spray-dried plasma, may increase growth performance, most likely due to an increased feed intake, probably due to its high palatability, but also due to the anabolic activity of IGF-I present in spray-dried plasma(Reference Van Dijk, Everts and Nabuurs218).
As nutrient composition and availability seem to be more important in the reparative phase(Reference Spreeuwenberg, Verdonk and Gaskins219), understanding how dietary factors can alter intestinal gene expression is an early step in the realisation of their therapeutic potential(Reference Sanderson and Naik220). The development of new strategies might involve stimulation of the immune response, reduction of the inflammatory response and improvement of nutrient digestion and transport. Improvement of the gut barrier function and mucosal repair by dietary factors might be other strategies to further improve health and animal performance. This can support intestinal health in times of disease or compromised feed intake such as at weaning. For such purposes, molecular nutritional science is of central interest. The response of mammalian organisms to changes in their nutritional environment may be detected at the mRNA and/or protein levels by different means, including expression arrays and proteome analysis, but also by use of transgenic approaches(Reference Stierum, Burgemeister and van Helvoort221). Such tools will allow identifying the mechanisms and pathways involved in the complex relationship between host factors and nutrition, but also the intestinal microflora.
Conclusions
In conclusion, dietary factors may have major impacts on intestinal gene expression, and deeper understanding of the underlying mechanisms and pathways is an early step in the realisation of their therapeutic potential. Stimulation or inhibition of genes by using specific functional constituents may be a convenient way to improve intestinal health under stressful conditions. For example, improvement of the gut barrier function and mucosal repair by dietary factors might be an interesting strategy to improve health and animal performance. In that way, intestinal health may be supported in times of disease or compromised feed intake such as at weaning. Furthermore, nutrients may be used by the intestinal microbiota resulting in the production of bacterial metabolites, which also may affect intestinal gene expression. Thus, modulation of the bacterial community by dietary ingredients, such as carbohydrate sources, can have sustainable effects on, for example, intestinal development and integrity. Since the microbiota affects a multitude of epithelial functions in the intestine due to specific bacterial–host interactions, the complex relationship between host, nutrition and intestinal microbiota has to be kept in mind when evaluating nutrition as a tool to influence gene expression.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
R. M., M. W. A. V. and E. B. were responsible for the conceptualisation and implementation of the manuscript. E. B. and B. U. M.-Z. wrote the manuscript. All authors reviewed the manuscript and approved it before submission. The authors wish to thank Ingrid Neff for assisting in preparing the manuscript.
There are no conflicts of interest.








