INTRODUCTION
Cervical cancer (CC) is one of the most common cancers in women worldwide with the highest incidence rates in developing countries. More than 120 types of human papillomavirus (HPV) have been identified and classified according to their oncogenic potential [Reference Bouvard1]. Although considerable controversy exists about the categorization of several low prevalent HPV types according to risk, the International Agency for Research on Cancer (IARC) has classified HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59, as group 1 high-risk oncogenic types [Reference Bouvard1]. Meta-analyses have shown that in all regions of the world, the majority of CCs are due to infection with HPV16 or 18, the first and second most prevalent HPV types, respectively [Reference de Sanjose2, Reference Li3]. However, considerable inter- and intra-regional variations have been reported for the distribution and role of other common high-risk types, namely HPV31, 33, 45, 52, 58 and 35. Although some differences may be accounted for by heterogeneity in typing methods and/or insufficient representation of some regions, certain geographical differences have been reported consistently across all meta-analyses.
The proportion of invasive cervical cancer (ICC) cases harbouring HPV16/18 worldwide is highest in North America (76·4%), followed by Europe (73·8%) and Africa (70%), while Asia (66·9%) and South America (65%) display a lower prevalence [Reference Smith4]. On the other hand, the prevalence of multiple infections is higher in developing countries in Africa (12·4%) and South/Central America (11%), than in countries in Asia (7·8%), Europe (6·2%) and North America (5·1%) [Reference Smith4].
Most studies have focused on geographical differences in HPV-type prevalence but in the United States [Reference Hariri5] and Malaysia [Reference Hamzi6] in-country differences in HPV prevalence across ethnic populations have also been reported. The relevance of HPV types other than HPV16/18 in CCs in specific regions and populations is especially relevant for targeted cancer prevention strategies and estimation of vaccination impact.
The two main approaches for CC prevention are HPV vaccination and oncogenic HPV detection during screening, allowing early treatment. Currently there is a bivalent and a quadrivalent HPV vaccine available. The impact of vaccination depends on the distribution of high-risk HPV types in a given population, since both vaccines target only high-risk types HPV16 and 18.
Suriname is a sparsely populated country in South America, where CC is among the leading causes of cancer-related deaths in women. The considerable contribution of HPV types other than HPV16/18 in CC in South America [Reference Ciapponi16] and the multi-ethnic population of Suriname accentuated the need for detailed HPV data especially in the context of the initiation of a national vaccination programme. Since 2013, the government has introduced HPV vaccination for schoolgirls aged 10–12 years, through a school-based vaccination programme.
The aim of this study was to determine the CC incidence, HPV prevalence and type-specific distribution in CC in the multi-ethnic population of Suriname in a 2-year period, shortly before the first vaccinations started.
METHODS
Study site
Suriname is situated along the North Coast of South America with the majority of the population of 541 638 persons (49·96% male, 50·04% female) [7] living in and around the capital Paramaribo. The population is highly diverse and consists of various distinct ethnic groups; 27·4% Hindustani, 21·7% Maroon (descendants of escaped West African slaves), 15·7% Creole (descendants of slaves who lived in the city during slavery or mixed primarily with Dutch Europeans), 13·7% Javanese, Amerindians (indigenous), Chinese, Caucasians (mostly descendants from Dutch and Portuguese Europeans) and others.
Study population and sample collection
Ethics approval was granted by the national Ethical Committee of the Ministry of Health (VG010-2012).
Archival, formalin-fixed paraffin-embedded biopsies of all women diagnosed with CC in Suriname in 2010 and 2011 (n = 111) were retrieved from the Pathology Department (Academic Hospital), the only Pathology facility in Suriname. These data therefore reflect the national situation. Samples from patients with a history of cervical conization or hysterectomy or with a diagnosis other than CC after confirmatory histological re-examination were excluded. From each formalin-fixed paraffin-embedded biopsy, 10-µm sections of tissue were sliced, adhering to a strict protocol to prevent cross-contamination of samples. The first and last sections of the biopsies were re-examined by an external pathologist to confirm the histopathological diagnoses. The sections in between were stored for DNA testing.
Laboratory testing
DNA was extracted from two 10-μm tissue sections with the QIAamp FFPE Tissue kit (Qiagen BV, The Netherlands) according to the manufacturer's protocol. The quality of extracted DNA was monitored through PCR amplification of the human β-globin gene. Specimens with a negative result were re-extracted with three 10-μm tissue sections. For samples remaining negative after two consecutive extractions, new sections were obtained from the paraffin block to repeat the extraction process.
HPV DNA was detected using a master-nested PCR combination utilizing the MY09/MY011 and GP05+/GP06+ primers [Reference Greer, Wheeler and Manos8]. HPV genotyping utilized a master E6/E7 PCR in combination with three multiplex PCRs [Reference Sotlar9], enabling the detection of 14 individual HPV types (high-risk types HPV16, 18, 31, 33, 35, 39, 45, 52, 56, 58, 59, the possibly carcinogenic HPV66 and low-risk types HPV6 and 11). Amplification products were detected on agarose or polyacrylamide gels after ethidium bromide staining. Negative and positive controls were included in each PCR run.
Data collection and analysis
Clinical records were traced in all hospitals and patients' information was anonymized. Patient characteristics, histopathological diagnosis and cancer type (squamous cell carcinoma/adenocarcinoma/clear cell carcinoma) were recorded.
Statistical analysis was performed using SPSS v. 21 (IBM Corp., USA). Because of the relatively small sample size in subgroups, differences between groups were analysed using non-parametric tests (Mann–Whitney test in continuous data and Fisher's exact test in proportions). For all tests P < 0·05 was considered statistically significant.
RESULTS
In 2010 and 2011, a total of 111 patients were diagnosed in Suriname with ICC, reflecting an age-standardized incidence rate of CC of 22·4/100 000 women. The majority of the cases were histopathologically classified as squamous cell carcinomas (91%), adenocarcinomas accounted for 9%, while no rare histological variants were detected.
One patient had an incomplete clinical record and two tissue specimens could not be retrieved.
All retrieved biopsies were confirmed as ICC upon re-examination, although 7% were reclassified mostly as squamous cell carcinoma instead of adenocarcinoma.
DNA extraction of the retrieved biopsies (n = 109) was not successful for 13 samples (12%), as attested by the PCR results, probably due to the inhibitory effect of remnants of paraffin, without significant variation in biopsies originating from 2010 and 2011. Samples with insufficient DNA yield were excluded. HPV DNA was detected in 91% of the valid samples.
General analysis of tumour type and analysis of age distribution was performed for all patients (n = 111), while analysis of HPV-type-specific distribution was performed on HPV-positive samples (n = 87). The HPV-type-specific distribution in CC cases presented in Table 1, shows 11 high-risk HPV types and the most common types, in descending order of frequency, were types 16, 18, 45, 66 and 58/52/35. The high-risk type HPV39 and low-risk types HPV6 and 11 were not detected in the CC samples. HPV16 was the most common type, with a presence of 25%. The contribution of HPV16 and 18 amounted to 43%. Worldwide, 87% of all CC cases are associated with the seven HPV types 16, 18, 45, 31, 33, 52 and 58 [Reference Muñoz10], while these high-risk HPV types account for just 64% of the CCs in this study.
Table 1. Distribution of HPV types in cervical cancer cases in Suriname (2010–2011)
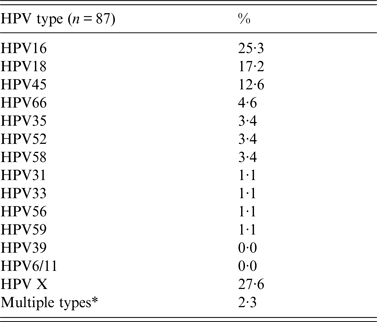
* Double infections (multiple types) have been scored for each HPV type.
The percentage of HPV positives not typed with the panel used, amounted to 28% (HPVX). The majority (97·7%) of the positive samples carried a single infection.
The HPV-type distribution seemed different for the two tumour types. Of the squamous cell tumours, the highest attribution was observed for HPV16, in contrast to the adenocarcinomas, mirroring the results from various other studies. However, the number of valid adenocarcinomas was rather small, precluding statistical analysis and therefore only overall data of ICC is discussed.
The mean age in the patient population (n = 111) was 53·2 years (range 27–91 years). The mean age was lower in HPV-negative women (52·3 years vs. 61·2 years, P = 0·266) than in HPV-positive women. The age distribution in the predominant HPV types is depicted in Figure 1.

Fig. 1. Age distribution in the predominant HPV types. HPV occurrence in percentages for four age groups.
The highest number of positive cases for HPV18 (53%) and HPV16 (41%) were noted in the 41–50 years age group. Women infected with HPV types 18 and 45 were on average 6 years younger at diagnosis than women with tumours associated with other HPV types. The majority of HPV16, 18 and 45 infections were found in women aged <50 years, in contrast to the other types.
Differences in HPV prevalence were observed in ethnic groups with a very high prevalence in Chinese and Amerindians, a lower prevalence in Javanese, and the prevalence dropped below 90% in descendants from immigrants from India and persons of African descent.
CC incidence was also significantly different between the various ethnic groups and is depicted against the population data in Figure 2. CC is more prevalent in Creoles, Javanese and Amerindians than in the major ethnic groups, Hindustani and Maroons.

Fig. 2. Cervical cancer cases (%) per ethnic group against the population data.
HPV distribution in the major ethnic groups is different as shown in Figure 3. Maroons displayed a higher percentage of HPV16 (50%) compared to the other ethnic groups (21%) (P = 0·038). Creoles displayed the highest genetic diversity with at least 10 different genotypes, whereas Maroons and Amerindians exhibited considerable less HPV genetic diversity.

Fig. 3. HPV-type distribution in the major ethnic groups. ‘Other’ includes HPV types 31, 33, 35, 52, 56, 58, 59, 66. HPV X corresponds to untyped HPV positives.
DISCUSSION
CC incidence
The age-standardized incidence rate of CC in 2010/2011 was 22·4/100 000 women, marking a decrease in Suriname within 20 years (26·7/100 000 women) [Reference Krul11], probably due to improved measures for early detection of pre-cancerous stages. This incidence rate is not only considerably higher than the estimated global crude incidence rate (14·0), but also places Suriname higher than neighbouring Brazil (17·3) and overall South America (20·4). On the other hand, this incidence is lower than neighbouring countries, French Guiana (29·1) and Guyana (44·7) [Reference Arbyn12], and more importantly significantly lower than the IARC estimates based on national mortality estimates using modelled survival [13]. Consequently, Suriname should be removed from the top 20 list of countries with the highest incidence of CC [14].
HPV overall prevalence and HPV-type prevalence
The HPV prevalence of 91% in ICC in Suriname is in line with the overall global presence (89·9%) [Reference Li3].
A high diversity of HPV types was exhibited with 11 high-risk HPV types detected in patients with CC. The absence of low-risk types HPV6 and 11, concurs with their minimal presence (0·9%) in cancers worldwide [Reference Li3]. The seven most common HPV types, in descending order of frequency, were types 16, 18, 45, 66 and 58/52/35. HPV16 and 18 are the first and second most prevalent types, corresponding with South American data and worldwide reports. However, the HPV genotype distribution in Suriname for the next most common types seems divergent from South America. HPV45 was the third most prominent type (13%) similar to Africa, North America, and Western/Central Asia [Reference Li3], while HPV31 as the third most common type in Central/South America (7·4%) [Reference Muñoz10] and Europe, was underrepresented with just 1%. HPV39, which seems almost entirely confined to Central/South America [Reference Bosch15] was not even detected. A relatively minor contribution was noted for HPV33, which consistently ranks in the top five in Central and Latin America and in the United States [Reference Ciapponi16, Reference Insinga17]. However, the third and fourth HPV types differ across regions and the amount of samples limited conclusions for less common HPV types.
Another interesting finding was the group 2B HPV66 type ranking ahead of HPV31 and 33, accentuating support for the reassessment of the carcinogenicity classification of HPV66, as was suggested in a recent study on biological activity of HPV66 in cancer tissue [Reference Halec18].
The predominance of HPV16 is consistent with other studies, but the HPV16 presence (25%) was markedly lower than the overall prevalence of 57·4% [Reference Muñoz10] ranging from 42% in Africa [Reference Ndiaye19] to 53·2% in Central/Latin America [Reference Ciapponi16] and even 96% in Greenland natives [Reference Sebbelov20]. However, low HPV16 presence has been observed earlier, especially in countries in South America (Chile 32%), Africa (Ghana 24%) and Asia (Japan 29·6%) [Reference Roa21–Reference Nagai23]. A contributing factor to the low HPV16 presence could be the multiplex PCR assay, with primers originally designed and tested on a European population, which might be less suitable for HPV16 subtypes or intratypic variants circulating elsewhere. On the other hand, another study utilizing several primer sets still yielded a HPV16 prevalence of 38% [Reference de Boer24], still well below the worldwide prevalence. Either, HPV16 has a less prominent role in CC in Suriname or high genetic variation in HPV16 may have caused mismatched primer binding. This notion is supported by a comparative study between The Netherlands and Suriname, reporting a higher occurrence of non-European HPV16 intratypic variants in Suriname [Reference de Boer24]. Furthermore, the intratypic HPV16 variants reported for Suriname seemed distinctively different from Central and South America. Explanations could be related to the different immigration patterns, leading to the highly multi-ethnic population in Suriname with an extensive potential for genetic diversity.
HPV18 was the second most common type (17%), consistent with international reports with an overall prevalence ranging from 13·2% in Latin America and the Caribbean to 20% in Oceania [Reference Li3, Reference Ciapponi16].
The joint contribution of HPV16 and 18 was significantly less frequent than in the rest of the world (43% vs. 70%, P < 0·001), due to the particularly low occurrence of HPV16.
The considerable differences implicated a divergent HPV genotype distribution in Suriname with a significant variation in the prevalence of some less common high-risk virus types and/or the presence of HPV16 variants. This premise is accentuated by the significantly higher percentage of unsubtyped cases than reported for Caribbean/Latin America (28% vs. 10%, P < 0·001). This result is most likely not due to the typing method, since the high percentage of untyped cases was corroborated in a recent study using a different method, a line probe assay targeting 25 different genotypes in Suriname, in which the unsubtyped cases of women visiting a STI clinic exceeded the persons infected with HPV16 [Reference Geraets25]. The data on epidemiology and physiopathogenesis of non-HPV16/18 types, subtypes and HPV16 intratypic variants is limited, but these results indicate a substantial impact on the aetiology of ICC in Suriname.
Only 2·3% of the samples harboured two HPV types, which is lower than the estimated 11·2% multiple infections in worldwide meta-analyses [Reference Li3]. On the other hand, considerable differences in multiple infections have been reported, ranging from 2·0% in Japan [Reference Nagai23] up to 95% in Syria [Reference Moustafa26]. One should take into account that the occurrence of multiple types may have been underestimated, due to the use of a panel with 14 HPV types. This seems unlikely, as the same assay could detect 22% multiple infections including triple infections in a previous study conducted in pre-cancerous women in Suriname (our unpublished data). The observed difference in multiple infections emphasizes the differences in HPV prevalence in pre-cancer stages and ICC. Further support was derived from the low occurrence of HPV18 (2% in an earlier study carried out in pre-cancerous patients in Suriname) vs. 17% HPV18 in this study (P < 0·001) and the predominance of HPV52 in women recruited at clinics in Suriname [Reference Geraets25], not manifested as ICC. The current results substantiate the finding that HPV types that preferentially progress to ICC differ from HPV types in asymptomatic women or women with low- or high-grade lesions [Reference Insinga17].
HPV distribution in relation to age
The mean age of women diagnosed with CC in Suriname (53·2 years) was higher than in Central/Latin America (50·7 years) and Asia (47·7 years) [Reference Alemany27], which may indicate delayed diagnosis, but could also be explained with the lower proportion of HPV16 in Suriname, since women infected with HPV16/18 are younger at diagnosis than women infected with other types. The higher age-specific prevalence observed in Suriname therefore also supports the importance of HPV16 intratypic variants or HPV types other than HPV16/18. The age distribution in the predominant HPV types displayed considerable differences (Fig. 1).
Women infected with HPV types 16, 18 and 45 were on average 6 years younger at diagnosis than women with tumours associated with other HPV types, a trend also observed in studies in England and Mexico [Reference Powell28, Reference Guardado-Estrada29]. HPV45, the third most common type in Suriname, was not even detected in the >60 years age group, consistent with other studies [Reference Murillo30]. The majority of HPV16, 18 and 45 infections occurred in women aged <50 years, with the reverse being true for the other types, in line with earlier observations [Reference Murillo30].
HPV presence in relation to ethnicity
HPV prevalence was highest for Chinese and Amerindians, while the prevalence dropped to 95% in Javanese and <90% in descendants of immigrants from India and persons of African descent. These results are in line with results from Malaysia where HPV prevalence was highest in Chinese (95·5%), followed by Malays (91·9%) and Indians (80%) [Reference Hamzi6].
The comparison of CC occurrence in ethnic groups set against the population data in Figure 2 revealed that Creoles, Javanese and Amerindians were significantly more prone to CC, while conversely CC was less prevalent in Hindustani and Maroons. The Javanese are also the major risk group in Suriname for hepatitis B infection [Reference Bänffer31]. On the other hand, this predominance is not displayed in the HIV prevalence [32], suggesting that rather than promiscuity, a genetic predisposition in Javanese may cause favouring of HPV virus persistence.
The pattern of HPV distribution in the major ethnic groups (Fig. 3) displayed considerable differences, as was also reported for multi-ethnic Malaysia [Reference Hamzi6]. Especially, the higher percentage of HPV16 (50%) in Maroons compared to the other ethnic groups (21%) (P = 0·047) was noteworthy. As could be expected from their secluded surroundings, the Maroons and the Amerindians, traditionally living in the interior, exhibited considerable less HPV genetic diversity than the Creoles with the highest variation of 10 HPV types. The absence of the less prevalent HPV types (other types) in the Maroons and Amerindians should be noted and the Maroons did not even harbour the third most common HPV45.
The higher occurrence of HPVX in Javanese and Amerindians coincided with their higher HPV positivity. This phenomenon could be explained since different types display variation in their oncogenic potential, inferring that HPVX either as other type, subtype or intratypic variant could also display a higher oncogenicity. Intratypic HPV16 variants have been reported with a transforming potential different from the HPV16 prototype [Reference Zehbe33].
Another interesting feature is the considerable difference between the ethnic groups in Suriname compared to their historical lineage. The HPV16 prevalence observed in Hindustani descendants from India, was 38%, while a HPV16 prevalence of 74% has been reported for India [Reference Bhatla34]. On the other hand, the results for HPV18 prevalence were similar (15% vs. 14%). The Javanese (descendants from Java) have a HPV16 count of 25%, while 34% has been reported for Jakarta (Java) [Reference De Boer35]. However, one should take into consideration that both the Hindustani and Javanese have a considerable contribution of untyped cases, 23% and 35%, respectively. The slave trade route can be traced back to the Ghana area and comparison of the prevalence of HPV16 between Maroons and people from Ghana shows an inverse relationship with only 24% HPV16 in Ghana [Reference Attoh22] vs. 50% in Maroons in Suriname. These results indicate that ethnic origin alone will not explain the disparities in HPV prevalence, although genetic predisposition can influence susceptibility to HPV infection and infection response. The differences in HPV prevalence in CC should be attributed to a complex interplay of multiple factors such as heritable factors, exposure, circulating HPV types, oncogenicity of specific HPV variants, environmental factors, cultural practices and also differential access to screening. Caution is warranted for the comparative analysis, because of substantial methodological variations in the different studies. Sequencing data will provide international comparability of results and more reliable recommendations for virological surveillance and for features required in new vaccines.
Implications for HPV vaccination efficacy
These data from a pre-vaccination period reflect the national situation of HPV-type distribution in confirmed CCs, the ultimate objective of cancer prevention programmes, and these results can therefore function as baseline to assess shifts in HPV genotype prevalence in Suriname after vaccination.
The demonstration that at least 11 different genital HPV types were associated with CC in Suriname has important implications for HPV vaccination as CC prevention strategy. The currently available vaccines Gardasil® (Merck & Co. Inc., USA) and Cervarix® (GSK, UK) protect against the high-risk types HPV16 and 18. The HPV vaccine efficacy for reducing CIN/2-3 lesions associated with HPV16 and 18 is more than 90%. Consequently, it has been estimated that vaccination can markedly reduce the burden of CC up to 71% worldwide.
However, in Suriname, the reduction of the annual burden of CC with Gardasil vaccination will range from 28% to 30%, with 70% vaccine coverage. This result is considerably lower than for South America, with a reduction of 55–69% (70% coverage) [Reference Goldie36] and The Netherlands, with an estimated 47% (50% vaccine coverage) [Reference Bogaards37]. It should be taken into account that the impact may be higher due to serological cross-protection against other types, since clinical vaccine trials were not designed to show efficacy in non-vaccine types. Moreover, some of the HPVX cases may be intratypic HPV16 variants with mutations prohibiting subtyping, but not necessarily evading immunological response, thus increasing anticipated vaccination impact.
Second-generation HPV vaccines include a nonavalent vaccine, additionally targeting HPV types 31, 33, 45, 52, and 58. Use of the nonavalent vaccine in Suriname could reduce the CC burden with an additional 15%, leading up to 45% in the case of 70% coverage, but would still not avert a substantial subset of CCs.
This anticipated low vaccination impact dictates that Suriname should implement additional preventive measures and provide sufficient information to communities to prevent misguided feelings of protection.
Cost-effectiveness analyses for continuation of vaccination should be performed also considering differences in vaccine impact per ethnic group. For instance, the higher percentage of HPV16 and 18 together in Maroons (75%) compared to the other ethnic groups (38%, P = 0·026), indicates that vaccination output in Maroons will surpass the impact in other ethnic groups.
Suriname and other multi-ethnic countries should tailor the information and measures presented to different ethnic communities based on the anticipated uneven impact in different ethnic groups. Furthermore, the substantial contribution of non-HPV16/18 types in ICC in Suriname highlights continued customized high-risk HPV screening as a prerequisite for CC reduction in these settings and the necessity for well-designed next-generation vaccines. These results add to the data challenging the impact of HPV vaccination for regions where the prevalence of HPV16/18 is less pronounced.
ACKNOWLEDGEMENTS
Research was conducted at the ‘Prof. Dr. Paul C. Flu’ Institute for Biomedical Sciences. We acknowledge the Ministry of Health (MOH) Suriname for facilitating this work. We also thank J. Kartowidjojo, J. Faerber, Sh. Pancham, M. Oron for the laboratory support and L. Nahar-van Venrooij for the statistical analysis.
This work was supported through the Ministry of Health of Suriname.
DECLARATION OF INTEREST
None.






