At present, more than two billion people worldwide, in particular in developing countries, are estimated to be deficient in essential vitamins and minerals, especially in vitamin A, Fe and Zn(1, 2). Micronutrient deficiencies occurring at particular stages of human life (pregnancy, breast-feeding, childhood) can severely affect health and development, leading in some cases to irreversible effects.
Fish can potentially contribute to reducing these micronutrient deficiencies. A few studies investigating this issue have been published in recent years. However, most of these studies are isolated stand-alone analyses, focusing on specific aspects of the problem. The overall contribution of fish to nutritional security is yet to be fully assessed. The purpose of the present study was to address this gap. Our main objective was to review and collate this scattered and relatively scarce literature in order to produce the first global overview of the role played by fish in improving nutrition in developing countries. In doing so, the quality of the data was carefully reviewed and data that lacked proper information on methods, units or sample size were excluded. Particular effort was made to highlight not only the information recently generated but also the gap in knowledge where more research is needed. Our focus was on developing countries where the largest proportion of people exposed to risk of undernutrition is found and where 95 % of the population depends on small-scale fisheries or small-scale aquaculture for their livelihood(3).
Understanding the nutritional importance of fish in developing countries
Fish as a major animal source of food in food-deficient countries
At the global level, fish consumption has increased from an average of 10·1 kg/capita per year in 1965 to 16·4 kg in 2005(4). However, the amount of fish consumption varies among regions. Figure 1 shows changes in fish consumption between 1965 and 2005 for different regions of the world. In all regions, fish consumption per capita has increased, except in sub-Saharan Africa and Latin America/the Caribbean, where it has stagnated from the early 1970s(4).
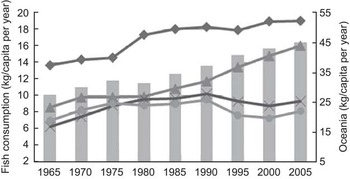
Fig. 1 Changes in fish consumption per capita (estimated by per capita availability of fish as food) for different developing regions (![]() , world;
, world; ![]() , Latin America and the Caribbean;
, Latin America and the Caribbean; ![]() , Oceania developing countries;
, Oceania developing countries; ![]() , South and South-East Asia;
, South and South-East Asia; ![]() , sub-Saharan Africa; data were calculated from the FAO food balance sheet(4))
, sub-Saharan Africa; data were calculated from the FAO food balance sheet(4))
FAO food balance sheets were used to estimate the contribution of fish to total protein and total animal protein at the country level(4). Among the thirty countries in the world where fish contribute more than one-third of the total animal protein supply (Fig. 2a), twenty-two are officially referred to as low-income food-deficient (LIFD) countries(5). In other words, a large majority (73 %) of the countries where fish is an important source of food are poor and food deficient. However, when other sources of protein (i.e. plant) are considered, the contribution of fish to total protein consumption is substantially low (Fig. 2b), indicating that in LIFD countries the majority of protein comes from plant-source foods. These statistics based on protein quantity at the national level, however, overlook the contribution of fish in terms of quality of protein and other nutrients.
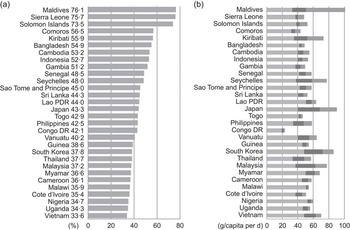
Fig. 2 (a) Fish as a percentage of animal protein consumption; (b) total protein consumption in g/capita per d (![]() , plant-source protein;
, plant-source protein; ![]() , other animal protein;
, other animal protein; ![]() , fish protein; data were calculated from the FAO food balance sheet(4))
, fish protein; data were calculated from the FAO food balance sheet(4))
Fish consumption pattern of the poor
Overall, data on fish consumption are scarce and poorly reflected in national statistics. However, the few field data that are available at household/community levels reveal the nutritional importance of fish among the poor. In Kapasia, Bangladesh, for example, the mean fish intake was as high as 83–96 g/person per d for whole fish in 1998–1999(Reference Thompson, Sultana and Nuruzzaman6), which was more than three times the national level (estimated by fish supply) in the same year(4). In Cambodia, surveys conducted in Svag Rineng province in 1997–1998 showed an average fish intake of 70 g/person per d for raw, cleaned parts (adjusted for cleaning loss of 30 % weight of raw, whole fish) and an intake of 9 g/person per d for raw, cleaned parts of other aquatic animals (n 66)(Reference Toft7), whereas the latest national statistics in Cambodia show only an average of 6·84 g/person per d(4). More recent surveys focusing on women in Kompong Chhnang, Prey veng and Kampong speu Provinces (Cambodia) suggest that the mean intake of fish could be as high as 103 g/d for raw, cleaned parts (n 163)(Reference Chamnan, Thilsted and Roitana8).
Regarding species consumed by the poor in rural areas in Asia, a variety of small indigenous species account for 50–80 % of the total amount of fish consumed(Reference Islam9–Reference Hels11). This higher dependence on smaller fish is explained by the fact that poor people can afford only comparatively cheaper fish species, whereas the better-off households purchase larger, medium-sized fish species, which they prefer because of the fact that they have fewer bones, more flesh and taste better(Reference Chamnan, Thilsted and Roitana8). In addition to wild small fish, data from Laos and Cambodia indicate that other aquatic animals such as frogs, freshwater molluscs and snails are frequently included in the everyday diet among the poor(Reference Chamnan, Thilsted and Roitana8, Reference Meusch, Yhoung-Aree and Friend12).
Similar trends seem to exist in sub-Saharan Africa. In Nigeria, household data indicate values of fish intake of approximately 217 g/person per d for whole fish in households in the inland state of Niger and 124 g/person per d for whole fish in the coastal state of Lagos(Reference Gomna and Rana13), whereas the national statistics indicate a consumption level of 24·6 g/person per d(4). In East Africa, the small indigenous species dagaa/omena/mukene (Rastrineobola argentea) from Lake Victoria is one relatively cheap fish that is consumed dried among the poor households(14–Reference Jansen16). Similarly, in Lubumbashi, Democratic Republic of the Congo (Congo DR), the small dried fish kapenta (Limnothrissa miodon) from Lake Kariba was reported to be the most consumed fish product by the lowest-income households(Reference Mujinga, Lwamba and Mutala17).
In general, one would expect the population that benefits from fish nutritional intake to be found along the coasts. However, data show that such benefits are also found among the inland population, as fish is a highly commercialised commodity that ‘migrates’ extensively within and between countries, even after it is caught. In sub-Saharan Africa, for instance, dried and smoked fish from Lake Chad travel more than 1000 km through a very efficient truck transportation network to be traded in urban markets in the south of Nigeria(Reference Ladu, Ovie and Erinne18). Similarly, in Ghana, smoked fish from coastal villages are traded as far as 600 km to Northern Ghana and sometimes even further north to Burkina Faso(Reference Overa19). In Lubumbashi, Congo DR, a large part of the smoked, dried and salted fish that are sold in markets come from Zambia, Tanzania, Zimbabwe, Malawi or even Mozambique(Reference Mujinga, Lwamba and Mutala17).
In summary, the data available from household surveys suggest that small fish, other aquatic animals and processed fish of low market value play a very important role in the diet of the poor not only through subsistence fishing but also through extensive market networks. The scattered evidence from the literature indicates an average fish intake of approximately 70–150 g/person per d for cleaned parts, confirming the nutritional significance of fish for the poor in Asia and sub-Saharan Africa.
The contribution of fish intake to improving nutrition
The contribution of fish to human nutrition and its impact on health have been examined from different perspectives in both developed and developing countries. In developed countries, the major focus has been on PUFA from fish and fish oil, which lowers blood pressure, reduces the risk of heart disease(Reference Wang, Harris and Chung20) and boosts infant growth and cognitive development(Reference Koletzko, Cetin and Brenna21). In contrast, in developing countries, focus has been on the role of fish in tackling undernutrition and micronutrient deficiencies.
Nutrients in fish
The nutrient content of fish varies with species. There are relatively limited data on the specific nutritional composition of the fish species consumed in developing countries, with some exceptions. In Bangladesh, the micronutrient content of approximately twenty small indigenous species has been examined and several nutrient-dense fish have been identified(Reference Thilsted, Roos and Hassan22, Reference Roos23). Partial information is also available on twenty-nine small indigenous species in Cambodia(Reference Roos, Chamnan and Loeung24, Reference Roos, Thorseng and Chamnan25). In north-east Thailand, fatty acid composition in five fish species and in a type of prawn that inhabits rice fields was established(Reference Karapangiotidis, Yakupitiyage and Little26). Table 1 summarizes the data found in the literature, grouped into three categories of differential nutritional significance: large freshwater fish, small freshwater fish and marine fish. Data for the latter group were extracted from the US Department of Agriculture(27). For the purpose of comparison, the nutrient content of some other food items is also displayed. Bold indicates high content values.
Table 1 The nutrient content of fish and other foods (per 100 g)Footnote *
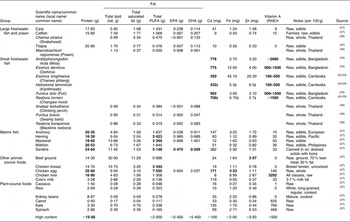
RAE, retinol activity equivalents.
Bold indicates high content values.
* Authors’ own compilation. Nutritional composition data from references listed.
† See Food and Nutrition Board, Institute of Medicine(61).
‡ Raw, cleaned parts.
Macronutrients in fish
Protein
Protein from fish contributes to the overall protein intake significantly as the digestibility of protein from fish is approximately 5–15 % higher than that from plants(28). Furthermore, protein from fish helps in the absorption of protein from plants. Staple foods such as rice or maize contain only a small amount of lysine, an essential amino acid, limiting the total absorption of protein. In contrast, animal sources of food such as fish have more balanced concentrations of all essential amino acids, and the concentration of lysine is particularly high(28). When fish is added to a plant-based diet, the total protein intake increases as lysine in fish compensates for the shortage of lysine in the rest of the diet. Therefore, fish play an important role in plant-based diets in LIFD countries.
Lipids
The lipid composition of fish is unique, having PUFA in the form of arachidonic acid (20 : 4n-6), EPA (20 : 5n-3) and DHA (22 : 6n-3), with many potential beneficial effects for adult health(Reference Wang, Harris and Chung20) and child development(Reference Koletzko, Cetin and Brenna21). The amount of PUFA in large freshwater fish such as carp and tilapia is relatively low, whereas the amount in smaller indigenous species is yet to be determined. Among fish species that are cheaper and traded in developing countries, small pelagic forage fish such as anchovies and sardines(Reference Tacon and Metian29) are perhaps some of the richest sources of PUFA(27).
Fish intake influences the PUFA levels in the breast milk of lactating women. In China the level of DHA in the breast milk of women living in coastal regions has been shown to be higher than the level in other regions(Reference Ruan, Liu and Man30). Similarly, in Tanzania, women with high intakes of freshwater fish had levels of arachidonic acid and DHA in their breast milk that were above the present recommendations for infant formulae(Reference Muskiet, van Goor and Kuipers31). However, it is still not clear how the PUFA in breast milk contributes to fetal and infant development, and further investigations are required into the quantities and nutritional significance of the fatty acids in fish species commonly consumed by the poor(Reference Roos, Wahab and Chamnan32, Reference Dewailly, Chateau-Degat and Suhas33).
Small fish as a source of micronutrients
Little attention has been given so far to the role of fish as a source of micronutrients. However, recent research suggests that small fish species that are consumed whole with bones, heads and viscera play a critical role in micronutrient intakes, as these parts are where most micronutrients are concentrated. Small fish also offer other nutritional advantages: they can be processed and stored for a long period; they are more affordable for the poor as they can be purchased in small quantities; and they can also be more evenly divided among household members(Reference Thilsted, Roos and Hassan22). The contribution of fish to micronutrient intakes is therefore determined not only by the nutrient content of the species but also by the local processing methods and eating patterns. As a consequence, several studies have indicated the actual nutrient content of the edible part by reflecting the local methods used to clean and prepare the fish for the meal (e.g. leaving or cutting off the head, removing a part of the viscera) and correcting the calculation for plate waste after meals(Reference Chamnan, Thilsted and Roitana8, Reference Roos23–Reference Roos, Thorseng and Chamnan25, Reference Roos, Wahab and Chamnan32) (see Table 2 for details).
Table 2 The micronutrient contents of small indigenous species in Bangladesh and CambodiaFootnote * (per 100 g, raw, edibleFootnote †)
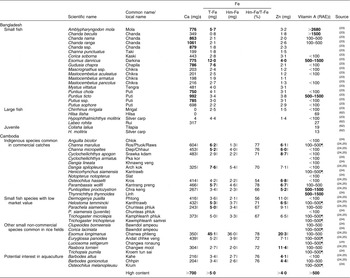
T-Fe, total Fe; Hm-Fe, haem Fe; RAE, retinol activity equivalents.
Bold indicates high content values.
* Authors’ own compilation. Nutritional composition data from references listed.
† Edible parts were estimated by employing local women to clean the fish according to traditional practices(Reference Roos23, Reference Roos, Wahab and Chamnan32).
‡ The Ca content in edible parts was calculated from plate waste in Bangladesh(Reference Roos23) (p. 53). The Ca content in Cambodian fish is calculated from raw, cleaned parts, except for E. longimanus, in which all fish bones were found to be consumed and therefore the Ca intake from fish was proportional to the measured Ca content(Reference Roos, Thorseng and Chamnan25) (p. 1231).
§ 1 RAE = 1 μg all-trans retinol = 1 RE (retinol equivalent)(61). All-trans 3,4-dehydroretinol and all-cis 3,4-dehydroretinol found in examined fish were calculated as having 40 % activity in relation to all-trans retinol, and 16 % activity in relation to β-carotene(Reference Roos23, Reference Roos, Wahab and Chamnan32).
∥ Raw, cleaned parts.
¶ Raw, whole fish.
Although small fish are rich in Ca and some forms of marine fish are rich in iodine, we focus specifically on vitamin A, Fe and Zn, deficiencies of which are widely spread and for which sustainable solutions have not been found yet.
Vitamin A
Dark-green, orange and yellow vegetables, which contain provitamin A carotenoids, have long been considered a major source of vitamin A and are often used in food-based interventions in order to increase vitamin A intake. However, as indicated in Table 1, some small fish are also very rich in vitamin A. In Bangladesh, two species, mola (Amblypharyngodon mola) and chanda (Parambassis baculis), were identified as having a vitamin A content as high as 2500 and 1500 μg retinol activity equivalents (RAE)/100 g raw edible parts, respectively(Reference Roos23). In Cambodia, the small indigenous species chanteas phluk (Parachela sianensis) and changwa mool (Rasbora tornieri) were reported to contain >1500 μg RAE/100 g raw edible parts(Reference Roos, Chamnan and Loeung24). Fish often exceed vegetables with regard to both the amount and frequency of consumption in some rural areas in Asia. For example, in Cambodia, children consume more fish than vegetables during all three seasons (mean intake 65·2 g/d per raw, cleaned parts for fish and 19 g/d for vegetables, n 163). At the household level, there is little difference in the amount of consumption between fish and vegetables (mean intake 654·6 g/d for raw, cleaned parts for fish and 765·1 g/d for vegetables) while fish are consumed more frequently than vegetables – 54·6 % of the surveyed households consumed fish 7 d/week, v. 47·9 % consumed vegetables(Reference Chamnan, Thilsted and Roitana8). In Kishoreganj, Bangladesh, field data show that daily consumption of small fish contributes 40 % of the total daily requirement of vitamin A at the household level(Reference Roos, Wahab and Hossain34). Finally, vitamin A being a fat-soluble vitamin, a small quantity of fat (e.g. 5 g fat/person per meal) is sufficient to ensure adequate bioefficacy(Reference Michaelsen, Hoppe and Roos35). Fish that are rich in vitamin A, cooked with some vegetables and some vegetable oils, are therefore an ideal combination to enhance vitamin A intake and bioefficacy.
There are two problems regarding vitamin A in fish. First, vitamin A content is species specific. The content may therefore be very different among fish species which belong to very close taxonomic groups. Similarly there is no direct relationship with habitat, and species living in the same habitat may have totally different vitamin A contents(Reference Hirao, Yamada and Kikuchi36). A former study that examined vitamin A in the flesh of 157 species found that nearly 85 % of the species contained little vitamin A (<60 μg RAE/100 g flesh), whereas a few others were extremely rich (e.g. >18 000 μg RAE/100 g flesh for the highest)(Reference Hirao, Yamada and Kikuchi36). Identifying particular species and promoting the consumption and conservation of those species continue to be a big challenge in many developing countries. Second, vitamin A in freshwater fish exists mainly in the form of 3,4-dehydroretinol. In Bangladesh, a study examined the efficacy of the intake of the local small fish mola in the daily diet (9 weeks, 6 d/week) in improving the vitamin A status of children measured through marginal serum retinol concentration in blood(Reference Kongsbak, Thilsted and Wahed37). However, no significant effect was found in the group of children fed fish curry, suggesting that vitamin A from mola, of which 80 % is 3,4-dehydroretinol, was not converted to retinol or was converted at an insufficient rate. Further studies on the effect of 3,4-dehydroretinol in man are clearly needed to confirm or refute this result, as well as to develop and test other indicators and approaches to assessing vitamin A status.
Fe
Some fish (especially small species) are rich in Fe (Tables 1 and 2); however, this nutrient is usually concentrated in the fish head and viscera. In a study conducted in Cambodia, the species chanwa phlieng (Esomus longimanus) was found to have a high content of Fe in its edible parts, even after the viscera had been removed through traditional cleaning methods. A serving of the sour soup made with this type of fish, eaten with boiled rice – the most common dish in the study area – was shown to supply on average 45 % of the daily requirement of Fe in women of childbearing age and 42 % of that in children(Reference Roos, Chamnan and Loeung24). Another important point with regard to Fe is the fact that the composition of Fe in fish is different from that found in plant-source foods: fish contain large amounts of haem Fe (Table 2), which is characterized by high bioavailability as opposed to non-haem Fe.
Zn
Cereals and legumes contain inhibitors of Zn absorption, such as phytate(38). The habitual diets of the poor, which are dominated by staple foods, therefore reduce Zn (as well as other minerals) bioavailability, and little Zn intake is expected from such diets. Yet, the daily Zn requirement in women in the third semester of pregnancy and among those lactating is as high as 20 mg/d(39). Likewise, children require 8·3–11·2 mg Zn/d depending on their body weight. These daily requirements are difficult to meet unless a significant amount of additional Zn is taken every day (unlike vitamin A, Zn cannot be stored in the human body(39) and is therefore needed in everyday diet).
Small fish are very rich in Zn compared with other animal-source foods and large fish species (Tables 1 and 2). The small low-market-value species chanwa philieng, for instance, which is commonly consumed by poor people in Cambodia, was found to contain 20·3 mg Zn/100 g raw edible part(Reference Roos, Thorseng and Chamnan25). A serving of the local sour soup dish contains on average 49 g of cleaned chanwa philieng, thus covering approximately 50 % of the daily Zn requirement for pregnant and lactating women(Reference Roos, Thorseng and Chamnan25). Another survey in the same country shows that fish contribute 33 % and 39 % of the total daily requirement of Zn in children and women, respectively(Reference Chamnan, Thilsted and Roitana8). Small fish in a plant-based diet is therefore expected to increase Zn intake considerably and to compensate for the low bioavailability induced by the phytate of the staple foods.
Although its deficiency is not prevalent, small fish consumed with bones are a very efficient source of Ca. Their bioavailability is as high as that of milk(Reference Hansen, Thilsted and Sandstrom40), whereas the concentration is approximately eight times higher than that of milk(Reference Larsen, Thilsted and Kongsbak41) (Table 2). According to a study conducted in Kishoreganj district in Bangladesh, an average daily small fish consumption of 65 g/person for edible part (cleaning and plate waste) can meet 31 % of the average daily requirement of Ca(Reference Roos, Wahab and Hossain34), whereas in Cambodia fish contribute 53 % of the daily requirement in children(Reference Chamnan, Thilsted and Roitana8). Small fish with bones are therefore introduced as a complementary food for children where milk is not available or affordable(Reference Gibson, Yeudall and Drost42).
Fish processing and its effects on the nutritional value of products
Nutritional loss through processing is a specific issue with fish, whereas that through cooking is common to many other foods including vegetables. Although in developing countries fish is often sun-dried, salted, fermented or smoked for preservation purposes, protein, fat and minerals remain stable even after processing. In contrast, vitamin A is sensitive to sunlight and heat. A study in Thailand found that boiled and sun-drying processing methods destroy 90 % of the vitamin A content in small fish. In contrast, steaming and oven-drying were shown to result in a 50 % loss only(Reference Chittchang, Jittinandana and Sungpuag43). In Bangladesh it was found that nearly all vitamin A in small fish is destroyed after sun-drying(Reference Roos23).
These results, however, need to be considered with a series of caveats. First, unlike other minerals and water-soluble vitamins, vitamin A can be stored in the human liver for 3–4 months(Reference Olson44). In Bangladesh and Cambodia, people mainly consume sun-dried fish only during the low-productive season(Reference Chamnan, Thilsted and Roitana8, Reference Roos23); therefore, vitamin A absorbed from small fish that are consumed fresh or with minimum processing will still be present in the human body weeks after the fish have been consumed, thus contributing to meeting long-term nutritional needs. Second, although they destroy certain vitamins, processing techniques still contribute greatly to extending the period during which fish can be consumed (by up to 4 or 5 months), thereby prolonging the time during which people who consume these processed fish can benefit from the remaining protein and micronutrients. Third, as mentioned above, loss of nutritional content through cleaning and cooking is not exclusive to fish. Vegetables that are boiled or fried also lose part of their nutritional value for the same reason.
The effects of fish in improving nutritional status
Fish as a complementary food for undernourished children
As part of the food-based approaches discussed in the literature, dietary diversification strategies suggest improving micronutrient intakes by promoting production and consumption of locally available nutritious foods(Reference Ruel45). In that context, utilising locally available small fish has considerable potential as a cost-effective food-based strategy to enhance micronutrient intakes. Earlier studies conducted in the 1990s were reviewed by Caulfield et al.(Reference Caulfield, Huffman and Piwoz46). Although fish was not the main focus in these experiments, recent studies in sub-Saharan Africa used small fish to increase intakes of Fe, Zn and Ca. In Malawi, for instance, Gibson et al.(Reference Gibson, Yeudall and Drost42) introduced fermented porridge mixed with whole-dried fish with bones and fruit as a complementary food. In addition, information on nutrition was also provided to mothers. After 12 months, the children in the intervention group showed lower incidence of common infectious illnesses and anaemia compared with control children, although no significant changes in hair Zn concentration and growth were found.
In Ghana, a study reported that fish powder from smoked anchovies mixed with fermented maize porridge supports rates of infant growth comparable to those obtained from a cereal–legume blend with vitamin- and mineral-fortified supplements, indicating the potential role of local fish in the improvement of infant growth(Reference Lartey, Manu and Brown47).
Finally, a study in Uganda used local dried fish mukene as an ingredient of low-cost supplement porridge to feed undernourished children. The experiment shows better outcomes in weight growth and mortality compared with the diets of imported skimmed milk that are usually used for undernourished children in hospitals(Reference Greco, Balungi and Amono48).
Challenges
Several challenges still remain in assessing and improving interventions on the contribution of fish to human nutritional status.
Measuring nutritional outcomes
Fish intake may improve the micronutrient content of a person's diet; yet, this does not necessarily mean that it will improve the nutritional status of that person or that this impact can be appropriately measured. Studies in Ghana, Malawi and Bangladesh, for instance, that tested the effect of fish intake on micronutrient status using biochemical indicators such as ferritin score, hair Zn concentration and retinol score found no statistically significant effects on the tested groups(Reference Kongsbak, Thilsted and Wahed37, Reference Gibson, Yeudall and Drost42, Reference Lartey, Manu and Brown47, Reference Lin, Manary and Maketa49), raising the question of whether appropriate indicators were used. However, the issue related to the choice of responsive nutritional outcomes to measure the impact of increased fish intake is not specific to fish; it is common to all experiments and interventions aimed at measuring the impact of changes in the intake of any food (e.g. meat intake, biofortified foods, etc.). Overall, problems continue to persist with regard to ‘demonstrating’ the impact on micronutrient status or other functional outcomes (infections, growth, etc.) and these problems apply to all food-based approaches, not only to fish-related ones. The reason for this is that the entire diet of the tested groups cannot be completely controlled, unless trials are conducted in clinical settings. The issue is that if people consume, say, more fish they may ‘compensate’ and eat less of other foods like chicken or other meat products. Demonstrating impact is therefore complex and does not depend only on having the right indicators; it also depends on having the right evaluation design. The latter, rather than the former, has been the biggest problem in evaluating food-based approaches up to now.
Sustainable supply of nutrient-dense fish
Data from Bangladesh and Cambodia confirm the potential of small indigenous species to improve nutritional status. For instance, for the vitamin A-rich small fish mola which is already present in 1·3 million of the small and seasonal fish ponds in Bangladesh, it was estimated that production of only 10 kg/pond per year could meet the annual recommended intake for two million children(Reference Roos, Islam and Thilsted10). From an ecological perspective, these small indigenous species are self-recruiting; that is, they reproduce and grow in natural water bodies, fish ponds and rice fields without breeding technology or any additional investment, although the productivity is usually low compared with fish aquaculture. Fish supplied by these common pool resources have, however, been reported to be declining in many areas in recent years. This trend may be one of the reasons for the decrease in fish consumption that has been observed, particularly among the rural poor, in some countries such as Bangladesh(14, Reference Roos, Chamnan and Loeung24, Reference Thompson, Roos and Sultana50), Laos(Reference Meusch, Yhoung-Aree and Friend12) or Malawi(Reference Dey, Kambewa and Prein51). Since it is not clear to what extent increased fish consumption through aquaculture can compensate for these declines (as aquaculture tends to favour the production of larger fish(Reference Kawarazuka and Béné52) with higher value in markets but lower nutritional value), there is a strong need to ensure that the fishing of small fish that takes place in common pool resources (ponds, floodplains, rivers) remains sustainable.
Addressing the underlying factors that determine the fish consumption patterns of the poor
Although fish is relatively cheap and accessible for the poor in many developing countries, recent studies in Asia indicate that low-income households consume less fish compared with rich households(Reference Islam9, Reference Dey, Rab and Paraguas53–Reference Bose and Dey55). Studies on socio-economic factors affecting Fe deficiency also show that the low-income group has lower intakes of Fe from fish and meat compared with better-off households, leading to a higher prevalence of Fe deficiency(Reference Bhargava, Bouis and Scrimshaw56, Reference Keskin, Moschonis and Dimitriou57). Many poor households do not have enough food stocks all year round, even for their basic needs of staple foods (energy deficits)(Reference Islam9, Reference Béné, Neiland and Jolley58, Reference Karim59). For these populations, fish caught by household members are often used to compensate for their requirement of staple foods to meet their daily energy needs. This responsive behaviour is one of the reasons why many countries in which people rely on fish as their main animal-source food are also low-income food-deficient countries. However, those are also the countries where micronutrient deficiencies are the highest and where interventions promoting fish as a nutrient-dense food are the most needed to maximize the benefits for the poor.
At the intra-household level, women and children, whose micronutrient needs are higher than those of men, unfortunately often consume less fish. For instance, a study in Nigeria found that male heads of households consumed 59 % more fish by weight compared with their wives and children and that when a single (large) fish was shared there was a tendency to distribute the body to the man, the tail to his wife and the head to the children on seven to eight out of ten occasions(Reference Gomna and Rana13). In Bangladesh, fish are likely to be distributed more evenly among household members compared with other animal-source foods; yet, adult men and pre-school boys still receive a larger share than their mother and/or sisters(Reference Bouis and Novenario-Reese60). Understanding social, economic and cultural contexts is therefore a necessary primary condition to improve the nutritional benefits accruing from fish for the population most in need.
Synthesis
The different sections above have summarized the information available on the nutrient contents of fish and the effects of processing them, as well as the bioavailability and efficacy of fish in improving the nutritional status of the poor. The main findings related to these points are summarized in Table 3, along with the areas in which requirements for further research on these issues have emerged.
Table 3 Summary of nutritional role by fish groups and gaps in knowledge

Conclusion
On account of its high nutrient content, fish could be used as a key component in strategies aimed at reducing micronutrient deficiencies in developing countries. A few studies have been published in relation to this issue; however, evidence is still sparse and fragmented and the overall potential contribution of fish to nutritional security is yet to be fully understood. The objective of the present study was to take the first step towards resolving this issue, by conducting a comprehensive overview of the existing literature and documenting more systematically the different nutritional roles that fish could play in preventing and controlling micronutrient deficiencies.
The review confirms that small indigenous species caught and/or traded locally do represent a major source of micronutrients in the everyday diet of the poor in developing countries because of their high nutrient content and bioavailability. Existing data suggest that adding fish to plant-based diets boosts protein absorption from food staples. Small fish, frequently consumed by the poor, are therefore nutritionally important, even in small quantities.
Currently, in most developing countries the production of small fish species is highly dependent on the activities of small-scale fisheries and to a lesser extent on aquaculture. A sustainable supply of these species should be prioritised and the production and consumption of nutrient-dense fish should be promoted in order to make full use of the capacity of these species in reducing micronutrient deficiencies. For this, conservation of wild stocks and dissemination of aquaculture techniques producing these small indigenous species are needed.
Further research is required in areas such as nutrient composition, cleaning/cooking methods, impact of declining fish catches on the fish consumption patterns of the poor and seasonal availability in order to derive appropriate policies and effective food-based approaches.
More technically, research protocols need to be developed to better quantify the outcomes of increased nutritional intake. In the specific case of fish, the present review revealed that so far very few studies have examined the nutritional outcomes of fish-related interventions. Research methods should also be developed to better monitor and assess aquaculture and small-scale fishery activities and their impact on the nutritional status of households.
Accumulating this type of information would certainly help in increasing our understanding of the mechanisms through which poor households can improve their dietary intake and nutritional status through fisheries and aquaculture activities, although we also recognise that primary causes of undernutrition might be multifaceted and that other factors such as market and income constraints, disease incidence and effects, health service provision and health environment, gender inequity and child care are likely to have equally important effects on nutritional status.
Acknowledgements
The present research was supported by the WorldFish Center. The authors have no conflict of interest to declare. N.K. conducted the initial literature search, whereas both authors contributed to the writing of the paper and therefore have primary responsibility for its final content. Institutional support from Helen Leitch and useful comments by Shakuntala H. Thilsted, Edward H. Allison and Marie Ruel on earlier drafts of the present paper are gratefully acknowledged.







