Introduction
Psychiatric disorders are characterized by heterogeneous clinical phenotypes thought to emerge from varied interconnected underlying pathophysiological mechanisms rather than monocausal pathways (Kendler, Reference Kendler2019). Evidence suggests that environmental and genetic factors are critical determinants of mental health and behavior (Smoller et al., Reference Smoller, Andreassen, Edenberg, Faraone, Glatt and Kendler2019). Moreover, the idea that environmental stressors could interact with hereditary predispositions in developing psychiatric conditions, the diathesis-stress model, has been debated for centuries (Kendler, Reference Kendler2020). More recently, this topic has been extensively explored in gene-environment interaction (GxE) studies. GxE studies aim to explore latent interactive effects between gene markers and environmental influences primarily to (1) provide insights into the etiology of the disease, (2) improve predictive models, and (3) identify subgroups more susceptible to environmental influences (McAllister et al., Reference McAllister, Mechanic, Amos, Aschard, Blair, Chatterjee, Conti, Gauderman, Hsu, Hutter, Jankowska, Kerr, Kraft, Montgomery, Mukherjee, Papanicolaou, Patel, Ritchie, Ritz and Witte2017). Although GxE studies have had a tremendous impact on the psychiatric literature, low replication rates have cast doubts to what extent these findings are valid (Border et al., Reference Border, Johnson, Evans, Smolen, Berley, Sullivan and Keller2019; Duncan & Keller, Reference Duncan and Keller2011). Inherently low statistical power in the candidate gene and candidate environment approach and challenges in the definition of environmental risk factors are among the reasons suggested to contribute to low replication rates (Duncan & Keller, Reference Duncan and Keller2011; McAllister et al., Reference McAllister, Mechanic, Amos, Aschard, Blair, Chatterjee, Conti, Gauderman, Hsu, Hutter, Jankowska, Kerr, Kraft, Montgomery, Mukherjee, Papanicolaou, Patel, Ritchie, Ritz and Witte2017). Of note, the idea that psychiatric disorders are associated with aggregate environmental risk factors, rather than any single factor, is well stablished since the groundbreaking research of Rutter et al (Rutter et al., Reference Rutter, Cox, Tupling, Berger and Yule1975, Reference Rutter, Tizard, Yule, Graham and Whitmore1976). Therefore, the proper investigation of GxE in psychiatric disorders may require the use of multivariable combined scores to represent both the liability conferred by genetic and environmental factors.
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent (Polanczyk et al., Reference Polanczyk, de Lima, Horta, Biederman and Rohde2007) neurodevelopmental condition with a suitable evidence profile for the study of GxE (Nigg et al., Reference Nigg, Nikolas and Burt2010). First, it is among the most heritable disorders across psychiatry, with a heritability index of 70% according to twin studies (Faraone & Larsson, Reference Faraone and Larsson2019). Second, its combined genetic effect can be appropriately represented using polygenic risk score (PRS) (Ronald et al., Reference Ronald, de Bode and Polderman2021). PRS for ADHD (ADHD-PRS) has been shown to predict ADHD diagnosis (Jansen et al., Reference Jansen, Dieleman, Jansen, Verhulst, Posthuma and Polderman2020) and dimensional traits in the population (Groen-Blokhuis et al., Reference Groen-Blokhuis, Middeldorp, Kan, Abdellaoui, Van Beijsterveldt, Ehli, Davies, Scheet, Xiao, Hudziak, Hottenga, Neale and Boomsma2014; Martin et al., Reference Martin, Hamshere, Stergiakouli, O’Donovan and Thapar2014). Third, several environmental factors have been consistently described to increase the risk for ADHD (Faraone et al., Reference Faraone, Banaschewski, Coghill, Zheng, Biederman, Bellgrove, Newcorn, Gignac, Al Saud, Manor, Rohde, Yang, Cortese, Almagor, Stein, Albatti, Aljoudi, Alqahtani, Asherson and Wang2021; Kim et al., Reference Kim, Kim, Lee, Jeong, Lee, Lee, Lee, Kronbichler, Stubbs, Solmi, Koyanagi, Hong, Dragioti, Jacob, Brunoni, Carvalho, Radua, Thompson, Smith and Fusar-Poli2020). Most are hypothesized to exert their effects by inducing neuronal damage during critical periods of development, with consequent disruptions of typical neurodevelopmental trajectories (Han et al., Reference Han, Patel, Jones and Dale2021). A recently published umbrella review performed a comprehensive and systematic evaluation of meta-analyses investigating the role of environmental risk factors in ADHD (Kim et al., Reference Kim, Kim, Lee, Jeong, Lee, Lee, Lee, Kronbichler, Stubbs, Solmi, Koyanagi, Hong, Dragioti, Jacob, Brunoni, Carvalho, Radua, Thompson, Smith and Fusar-Poli2020). Eight environmental factors were deemed to have convincing or highly suggestive evidence of association with ADHD, and a further 23 environmental factors were considered as having suggestive or weak evidence of association (Kim et al., Reference Kim, Kim, Lee, Jeong, Lee, Lee, Lee, Kronbichler, Stubbs, Solmi, Koyanagi, Hong, Dragioti, Jacob, Brunoni, Carvalho, Radua, Thompson, Smith and Fusar-Poli2020).
Although the role of both genetic and environmental factors on the pathophysiology of ADHD has been extensively explored, little is known regarding their interactive effects in disorder development (Nigg et al., Reference Nigg, Nikolas and Burt2010). Studies exploring GxE were almost solely conducted using a candidate gene approach (Nigg et al., Reference Nigg, Nikolas and Burt2010), and significant interactions findings were usually succeeded by failed replication attempts (Nigg et al., Reference Nigg, Nikolas and Burt2010). Moreover, prior studies investigating GxE have consistently focused on a single environmental factor (Nigg et al., Reference Nigg, Nikolas and Burt2010), which have limited power to detect significant interactions since environmental factors appear to increase the risk for ADHD in an additive manner (Schmitt & Romanos, Reference Schmitt and Romanos2012). Additionally, there is heterogeneity in the risk (both environmental and genetic) and resilience factors contributing to the emergence of ADHD symptoms in each individual (Faraone et al., Reference Faraone, Banaschewski, Coghill, Zheng, Biederman, Bellgrove, Newcorn, Gignac, Al Saud, Manor, Rohde, Yang, Cortese, Almagor, Stein, Albatti, Aljoudi, Alqahtani, Asherson and Wang2021). Furthermore, as far as we are concerned, no study has explored GxE in ADHD by combining environmental risks into a single score. Therefore, in this study, we aimed to: (1) evaluate whether environmental and genetic factors have a synergistic effect in increasing symptoms of ADHD using ADHD-PRS to represent the combined influence of genetics, and environmental risk score (ERS) to represent the combined influence of the environment; (2) test the specificity of our findings by exploring interactive effects between ADHD-PRS and ERS for ADHD in depressive and anxiety symptoms; and (3) investigate the impact of environmental and genetic factors in the progression of ADHD over time. Our primary hypothesis was that environmental and genetic factors would act synergistically to increase the phenotypic expression of ADHD symptoms.
Methods and materials
Sample
Participants were children and adolescents (6 to 14 years of age on baseline) from the Brazilian High-Risk Cohort Study for Psychiatric Disorders (BHRCS), a large school-based community cohort designed to study developmental trajectories of psychopathology and mental disorders (Salum et al., Reference Salum, Gadelha, Pan, Moriyama, Graeff-Martins, Tamanaha, Alvarenga, Valle Krieger, Fleitlich-Bilyk, Jackowski, Sato, Brietzke, Polanczyk, Brentani, de Jesus Mari, Do Rosário, Manfro, Bressan, Mercadante and Rohde2015). The BHRCS started in 2010 when 9,937 children were selected from 57 schools in Porto Alegre and São Paulo and submitted to a first screening. This was followed by a selection of a high-risk subgroup for psychiatric disorders composed of 1,553 participants with psychiatric symptoms and high family loading of symptoms, and a randomly selected subgroup of 958 individuals. Details of the high-risk selection procedures can be found elsewhere (Salum et al., Reference Salum, Gadelha, Pan, Moriyama, Graeff-Martins, Tamanaha, Alvarenga, Valle Krieger, Fleitlich-Bilyk, Jackowski, Sato, Brietzke, Polanczyk, Brentani, de Jesus Mari, Do Rosário, Manfro, Bressan, Mercadante and Rohde2015). From those 9,937 children, 2,511 have been further assessed in three time points: baseline (2010–2011), wave 1 (2013-2014), and wave 2 (2018–2019), with retentions rates of 80 and 75%, respectively.
Procedures taken during this study followed the Helsinki Declaration of 1975, as revised in 2008. All participants were informed and gave written (parents) and verbal informed consent (subjects younger than 18 years of age). Since procedures involved human subjects, all methods were approved by the institutional review boards of all institutions engaged in the study (CAAE: 74563817.7.1001.5237).
Outcome measures
Dimensional symptoms
In each of the three evaluations, the Child Behavior Checklist (CBCL) (Bordin et al., Reference Bordin, Rocha, Paula, Teixeira, Achenbach, Rescorla and Silvares2013) and the Strength and Difficulties Questionnaire (SDQ) (Goodman, Ford, Simmons, et al., Reference Goodman, Ford, Simmons, Gatward and Meltzer2000) were used as a measure of dimensional psychopathology. For individuals older than 18 at the third evaluation, the Adolescent Behavior Checklist (ABCL) (Adams et al., Reference Adams, Kelly and McCarthy1997) was used instead of the CBCL. The CBCL and the ABCL are parent-report questionnaires that assess emotional, behavioral, and social problems. The SDQ is a 25-item parent-report questionnaire that provides four risk groups of behavioral and emotional difficulties (Goodman, Ford, Simmons, et al., Reference Goodman, Ford, Simmons, Gatward and Meltzer2000). As a measure of ADHD symptoms, we used raw scores derived from the Attention Problems subscale of CBCL or ABCL (Adams et al., Reference Adams, Kelly and McCarthy1997; Bordin et al., Reference Bordin, Rocha, Paula, Teixeira, Achenbach, Rescorla and Silvares2013) and raw scores from the Hyperactivity subscale of SDQ (Goodman, Ford, Simmons, et al., Reference Goodman, Ford, Simmons, Gatward and Meltzer2000). To measure depressive/anxiety symptoms, we used raw scores from the Anxious/Depressed subscale of CBCL or ABCL (Adams et al., Reference Adams, Kelly and McCarthy1997; Bordin et al., Reference Bordin, Rocha, Paula, Teixeira, Achenbach, Rescorla and Silvares2013) and raw scores from the SDQ Emotion Scale (Goodman, Ford, Simmons, et al., Reference Goodman, Ford, Simmons, Gatward and Meltzer2000).
Psychiatric diagnosis
In each of the three evaluations, the diagnosis of psychiatric disorders (ADHD, depressive disorders, and anxiety disorders) according to DSM-IV was performed using the Development and Well-Being Assessment (DAWBA) (Goodman, Ford, Richards, et al., Reference Goodman, Ford, Richards, Gatward and Meltzer2000), a validated structured interview administered by trained lay interviewers. The rating procedure was performed by nine certified child psychiatrists trained and supervised closely by a senior child psychiatrist with extensive experience rating the DAWBA. The Brazilian Portuguese version showed acceptable indexes, with agreements ranging from 90 to 95%, and Kappa from 0.72 to 0.84 (Salum et al., Reference Salum, Gadelha, Pan, Moriyama, Graeff-Martins, Tamanaha, Alvarenga, Valle Krieger, Fleitlich-Bilyk, Jackowski, Sato, Brietzke, Polanczyk, Brentani, de Jesus Mari, Do Rosário, Manfro, Bressan, Mercadante and Rohde2015). For the diagnosis of ADHD, DAWBA assessed the 18 ADHD symptoms, their pervasiveness, age at onset, and associated impairment. A similar approach was used for ascertaining the diagnosis of depressive disorders (including major depressive disorder, other specified depressive disorder, and unspecified depressive disorder) and anxiety disorders (including separation anxiety, specific phobia, social phobia, panic disorder, agoraphobia, post-traumatic stress disorder, obsessive-compulsive disorder, generalized anxiety disorder, and other specified anxiety).
Polygenic risk scores
Genomic DNA was isolated from saliva (Oragene) using prepIT-L2P reagent (DNAgenotek). Genotyping was performed using the Global Screening Array (Illumina). Single-nucleotide polymorphisms (SNPs) with a minor allele frequency <1%, locus missingness >10%, or Hardy-Weinberg equilibrium significance <.000001 were excluded, as were individuals with genotype missingness >10% and an estimation of identity by descent >0.12.
ADHD-PRSs were calculated as the weighted sum of risk alleles for ADHD according to the most recent genome-wide association study (GWAS) (Demontis et al., Reference Demontis, Walters, Martin, Mattheisen, Als, Agerbo, Baldursson, Belliveau, Bybjerg-Grauholm, Bækvad-Hansen, Cerrato, Chambert, Churchhouse, Dumont, Eriksson, Gandal, Goldstein, Grasby and Grove2019). The calculation was performed using the PRSice software v2 (Euesden et al., Reference Euesden, Lewis and O’Reilly2015). P-value-informed clumping was performed, retaining SNPs with the smallest P-value within a 250-kb window and excluding those SNPs in linkage disequilibrium (r 2 > 0.1). Nine ADHD-PRSs were calculated using subsets of SNPs selected according to the following GWAS p-value thresholds: 1, 0.8, 0.5, 0.4, 0.3, 0.2, 0.1, 0.05, 0.01. The total number of SNPs in each threshold can be found in Table S1. Main analyses were performed with the threshold of 1, which included all SNPs. ADHD-PRSs were transformed into z-scores for better visualization. To investigate population structure, principal components analysis was conducted using PLINK 1.9 (Chang et al., Reference Chang, Chow, Tellier, Vattikuti, Purcell and Lee2015).
Environmental risk scores
ADHD ERSs were calculated using an approach adapted from PRS, as previously performed for schizophrenia (Padmanabhan et al., Reference Padmanabhan, Shah, Tandon and Keshavan2017). In summary, we identified the most consistent environmental factors associated with ADHD according to the literature. We extracted and log-converted the odds ratio estimated in the meta-analyses to obtain beta values. Then, we multiplied the beta of each environmental risk by 1 if the exposure was present in a particular subject or by 0 if it was absent, and calculated the weighted sum for each individual. The selection of environmental factors was performed in a two-step approach: (1) we identified all potential environmental risk factors reported on the recent and comprehensive umbrella review of meta-analyses published by Kim et al., (Kim et al., Reference Kim, Kim, Lee, Jeong, Lee, Lee, Lee, Kronbichler, Stubbs, Solmi, Koyanagi, Hong, Dragioti, Jacob, Brunoni, Carvalho, Radua, Thompson, Smith and Fusar-Poli2020), in which authors systematically evaluated meta-analyses of environmental risk and protective factors for ADHD; (2) we performed a literature review to identify potentially relevant meta-analyses published after October 2019 (date of search by Kim et al. (Reference Kim, Kim, Lee, Jeong, Lee, Lee, Lee, Kronbichler, Stubbs, Solmi, Koyanagi, Hong, Dragioti, Jacob, Brunoni, Carvalho, Radua, Thompson, Smith and Fusar-Poli2020)). A detailed description of included and excluded environmental risk factors identified in each step, as well as reasons for exclusion, can be found in the Supplementary Material. The environmental risk factor was included in our score if it: (1) had been reported as OR or RR (for the prevalence of ADHD, both values are similar) (Cummings, Reference Cummings2009); (2) was statistically significant in a random-effects summary estimate; (3) was assessed by a minimum of three estimates; and (4) had been collected in our sample. If authors reported adjusted and unadjusted effect estimates, the adjusted estimates were extracted even if estimated from fewer studies. If more than one meta-analysis evaluating the same risk factor was found, the one with more studies included was selected for extraction, considering that both followed similar methodological criteria. Environmental risk factors included in our score can be found in Table S2. Risk factors not included in the score can be found in Table S3.
Statistical analysis
Mixed-effects linear models were performed to test the interaction between ADHD-PRS and ERS on ADHD symptoms (CBCL/ABCL, SDQ). As a secondary analysis, mixed-effects logistic regression models were performed to test the interaction between ADHD-PRS and ERS on ADHD diagnosis. We decided to use continuous measurements of symptoms in our primary analysis, rather than the categorical diagnosis, to increase the statistical power. The following model was used: ADHD symptoms ∼ ADHD-PRS*ERS + ADHD-PRS + ERS + covariates. We also used mixed-effects linear models and mixed-effects logistic regression models to confirm the association between ADHD-PRS and ADHD while controlling for ERS, and vice-versa (ADHD symptoms ∼ ADHD-PRS + ERS + covariates). Additionally, we tested the interaction between age and ADHD-PRS, and between age and ERS. This was performed to evaluate whether ADHD-PRS or ERS were associated with distinct trajectories over time. For that, the following models were used: ADHD symptoms ∼ ADHD-PRS*age + ADHD-PRS + age + covariates, and ADHD symptoms ∼ ERS*age + ERS + age + covariates. All models were adjusted for sex, age, and ancestry (using the first ten principal components). Furthermore, as recommended for proper adjustment, we adjusted for the interaction between the continuous independent variables (ADHD-PRS, ERS) with sex and age (Keller, Reference Keller2014). Data from the three assessments were used, and mixed models were fit including subject-specific random slopes and intercepts to cluster the multiple assessments per individuals. ADHD-PRS, ERS, and dimensional psychopathology measures were standardized. Our primary analyses were conducted including all SNPs (ADHD-PRSs with a threshold of 1). Sensitivity analyses with additional thresholds were performed, and they can be found in the Supplementary Material.
To confirm the specificity of our findings, similar models were fit using symptoms of depression and anxiety, as well as their categorical diagnosis. A linear regression model was performed with ERS as the dependent variable and ADHD-PRS as the independent variable to test for gene-environment correlation. Logistic regression models were also performed using each environmental factor as a dichotomic dependent variable (yes/no) and ADHD-PRS as the independent variable. Significant interactions were explored using marginal effects to represent the change in linear prediction of an outcome for one standardized unit change of ADHD-PRS when ERS is held constant at different values (z-scores of −2 to 2, with 1-unit increases). Comparisons of demographic characteristics between individuals with or without ADHD were performed using χ2 tests for categorical variables and t-tests for continuous variables. The percentage of variance in symptoms explained by genetic and environmental factors was calculated using the Bosker/Snijders pseudo R 2 value for mixed-effects linear models (Snijders & Bosker, Reference Snijders and Bosker1994). The variance explained by GxE was calculated by subtracting the variance explained by the model with ADHD-PRS and ERS (ADHD symptoms ∼ ADHD-PRS + ERS + covariates) from the variance explained by the full model (ADHD symptoms ∼ ADHD − PRS*ERS + ADHD-PRS + ERS + covariates). P-values lower than 0.05 were considered statistically significant. All analyses were performed using Stata version 14.0 (StataCorp, College Station, Texas USA). Graphs were performed using GraphPad Prism version 8.4.0 (GraphPad Software, California, USA).
Results
From the 2,511 individuals evaluated at baseline, GWAS data was available for 2,189, and complete data (including genotyping, environmental risk factors, and psychiatric diagnosis) was available for a total of 2,046 individuals. At baseline, the mean (SD) age was 10.18 (1.91) years, and 940 (45.94%) of the subjects were female (Table 1). From the 2,046 individuals with complete data at baseline, 1,685 were followed in wave 1 (mean age = 13.44; SD = 1.91) and 1,603 in wave 2 (mean age = 18.27; SD = 2). The prevalence of ADHD was 11.49% at baseline, 5.22% in wave 1, and 2.81% in wave 2 (Table 1). Demographic characteristics at each assessment can be found in Table 1. Sample characteristics for individuals with or without a diagnosis of ADHD at each time point can be found in the Supplementary Material (Tables S4, S5, and S6). In sensitivity analyses, we observed decreased maternal education (defined as no education or elementary school only) to be consistently associated with lower rates of follow-up (Table S7). Furthermore, we observed that higher maternal age and a diagnosis of anxiety at baseline were consistently associated with higher rates of follow-up (Table S7).
Table 1. Demographic characteristics at each assessment
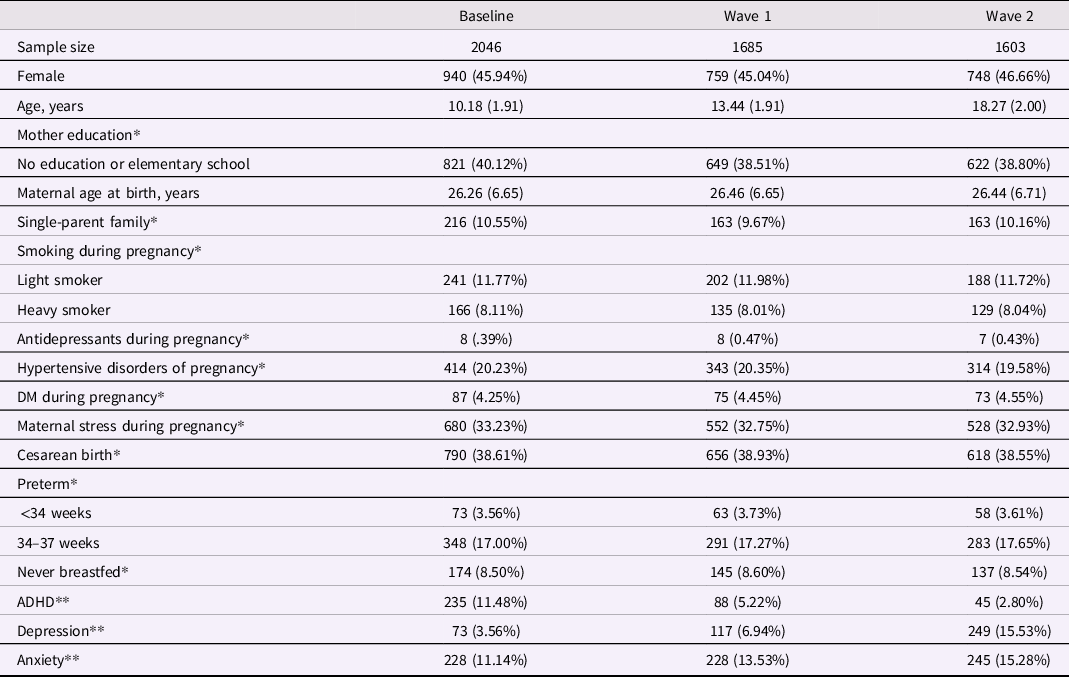
DM = diabetes mellitus; ADHD = attention-deficit/hyperactivity disorder.
Continuous variables are presented as mean and standard deviation.
*Data collected only at baseline.
**Data collected at each assessment.
Environmental risk factors
The following environmental risk factors were included in our ERS: (1) low maternal education (Russell et al., Reference Russell, Ford, Williams and Russell2016); (2) low maternal age (Min et al., Reference Min, Li and Yan2021); (3) single-parent family (Russell et al., Reference Russell, Ford, Williams and Russell2016); (4) smoking during pregnancy (Huang et al., Reference Huang, Wang, Zhang, Zheng, Zhu, Qu and Mu2018); (5) use of antidepressants during pregnancy; (6) hypertensive disorders of pregnancy (Maher et al., Reference Maher, O’Keeffe, Kearney, Kenny, Dinan, Mattsson and Khashan2018); (7) diabetes mellitus during pregnancy (Zeng et al., Reference Zeng, Tang, Yue, Li, Qiu, Hu, Tang, Wang, Yang, Qu and Mu2019); (8) maternal stress during pregnancy (Manzari et al., Reference Manzari, Matvienko-Sikar, Baldoni, O’Keeffe and Khashan2019); (9) cesarean delivery (Zhang et al., Reference Zhang, Sidorchuk, Sevilla-Cermeno, Vilaplana-Perez, Chang, Larsson, Mataix-Cols and Fernandez de la Cruz2019); (10) preterm birth (Allotey et al., Reference Allotey, Zamora, Cheong-See, Kalidindi, Arroyo-Manzano, Asztalos, van der Post, Mol, Moore, Birtles, Khan and Thangaratinam2018; Franz et al., Reference Franz, Bolat, Bolat, Matijasevich, Santos, Silveira, Procianoy, Rohde and Moreira-Maia2018); and (11) absence of breastfeeding (Yan Zeng et al., Reference Zeng, Tang, Tang, Shi, Zhang, Zhu, Xiao, Qu and Mu2020) (for a detailed description of the selected environmental risk factors, please see the Supplementary Material). Data regarding environmental risk factors were obtained during the household parent interview at baseline. No evidence of gene-environment correlation was detected, with OR ranging from .96 to 1.10 (Table S8).
ADHD
Mixed-effects linear regression models demonstrated a significant interaction between ADHD-PRS and ERS on CBCL/ABCL Attention Problems and on SDQ Hyperactivity (Figure 1, Table 2). Marginal effect analysis revealed a positive association between ADHD-PRS and symptoms of ADHD only in individuals with ERS z-score > 0. The strength of the association between symptoms of ADHD and ADHD-PRS increased as a function of growing ERS. For example, at ERS of 0 z-score, each standardized unit increase in ADHD-PRS was associated with an increase of .08 z-score on CBCL/ABCL Attention Problems (95% CI .04, .12; p < .0001), and .07 z-score on SDQ Hyperactivity (95% CI .04, .11; p < .0001). At ERS of 2 z-scores, each standardized unit increase in ADHD-PRS was associated with an increase of .17 on CBCL/ABCL Attention Problems (95% CI .10, 0.25; p < .0001), and .15 on SDQ Hyperactivity (95% CI .08, .23; p < .0001). There was no association between ADHD-PRS and symptoms of ADHD in individuals with ERS of -1 z-score (each standardized unit increase in ADHD-PRS was associated with an increase of .037 [95% CI −.015, .090; p = .16] and .039 [95% CI −.013, .093; p = .14], for CBCL/ABCL Attention Problems and SDQ Hyperactivity, respectively). Similarly, no association was found for ERS of -2 z-scores (each standardized unit increase in ADHD-PRS was associated with a decrease of .008 [95% CI −.08, .07; p = .82] and of .0004 [95% CI −.08, .08; p = .99], for CBCL/ABCL Attention Problems and SDQ Hyperactivity, respectively). Similar findings were obtained after correcting for sample attrition using inverse probability weights (Table S9). In sensitivity analyses excluding one factor at a time from the ERS, results were still significant except when the ERS was calculated excluding maternal stress during pregnancy or maternal education (Table S10).
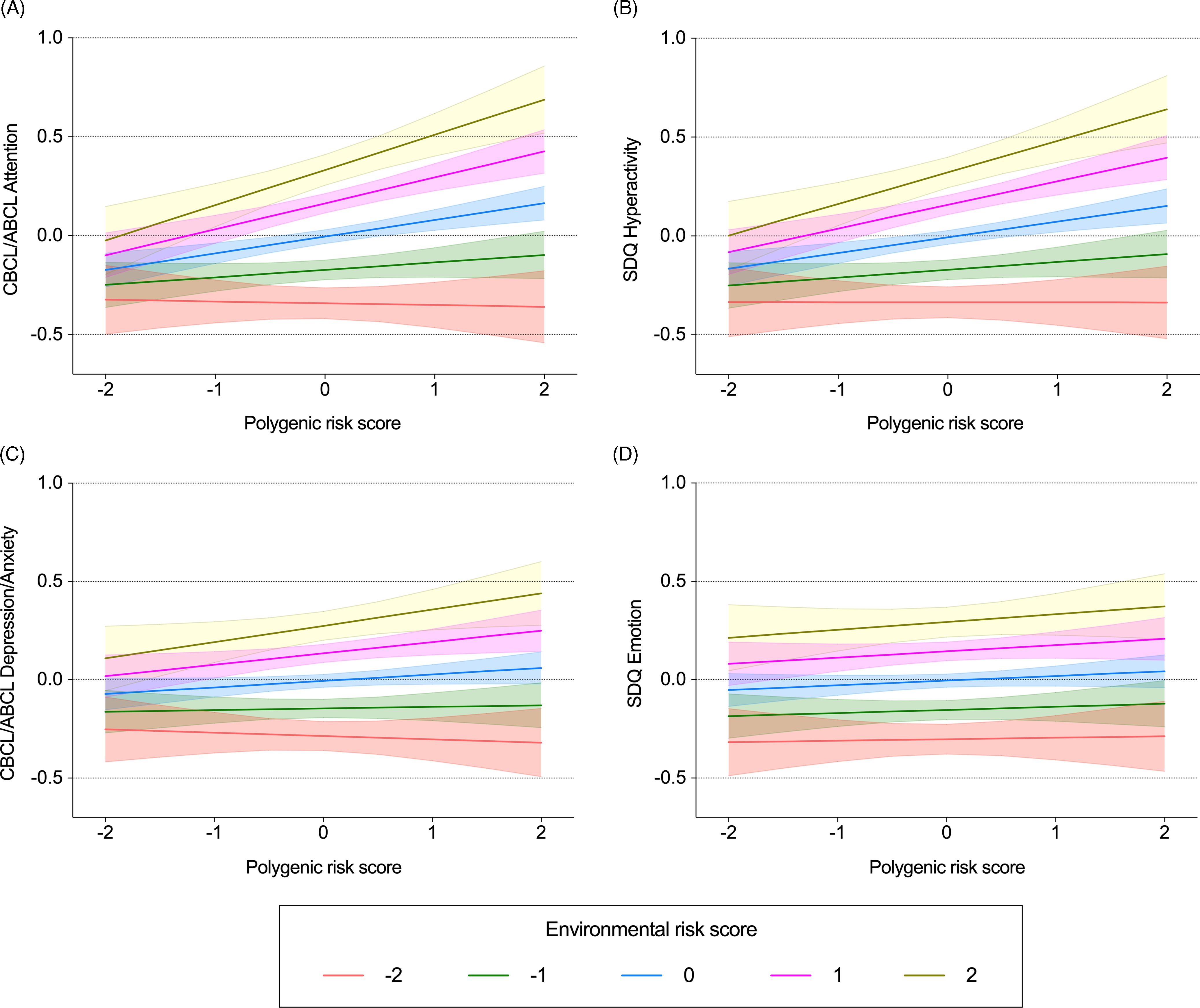
Figure 1. Interactive effects between polygenic risk scores and environmental risk scores on dimensional psychopathologic measures. Mean predicted values and 95% confidence intervals of dimensional symptoms of attention-deficit/hyperactivity disorder (ADHD) (A, B) and depression/anxiety (C, D) estimated from linear mixed-effects models testing the interaction between ADHD-PRS and ERS. Data are presented as z-scores.
Model = Dimensional symptoms ∼ ADHD-PRS*ERS + ADHD-PRS + ERS + age + sex + age*ADHD-PRS + age*ERS + sex*ADHD-PRS + sex*ERS + 10 PCs.
CBCL = Child Behavior Checklist; ABCL = Adolescent Behavior Checklist; SDQ = Strength and Difficulties Questionnaire; ADHD = attention-deficit/hyperactivity disorder; PRS = polygenic risk score; ERS = environmental risk score; PCs = principal components.
Table 2. Main effects and interactions between environmental risk scores and polygenic risk scores on dimensional psychopathologic measures
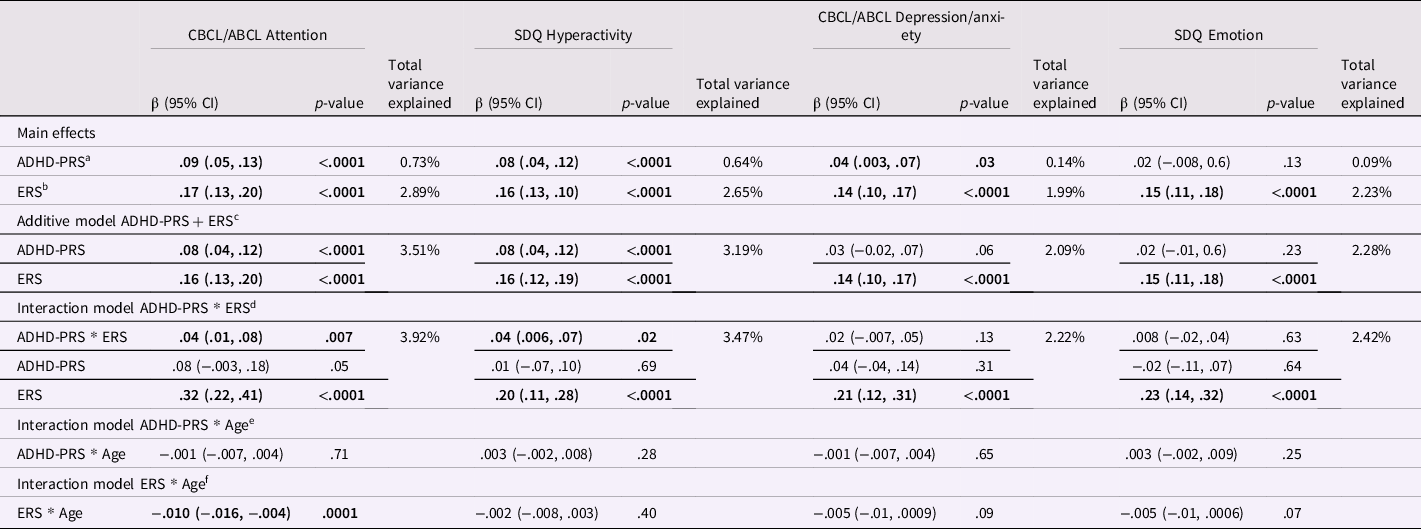
β = regression coefficient from the mixed-effects linear regression models; CI = confidence interval; CBCL = Child Behavior Checklist; ABCL = Adolescent Behavior Checklist; SDQ = Strength and Difficulties Questionnaire; ADHD = attention-deficit/hyperactivity disorder; PRS = polygenic risk scores; ERS = environmental risk score; PCs = principal components.
a. Model = Dimensional symptoms ∼ ADHD-PRS + age + sex + 10 PCs.
b. Model = Dimensional symptoms ∼ ERS + age + sex.
c. Model = Dimensional symptoms ∼ ADHD-PRS + ERS + age + sex + 10 PCs.
d. Model = Dimensional symptoms ∼ ADHD-PRS*ERS + ADHD-PRS + ERS + age + sex + age*ADHD-PRS + age*ERS + sex*ADHD-PRS + sex*ERS + 10 PCs.
e. Model = Dimensional symptoms ∼ ADHD-PRS*age + ADHD-PRS + age + sex + sex*ADHD-PRS + sex*age + 10 PCs.
f. Model = Dimensional symptoms ∼ ERS*time + ERS + age + sex + sex*ERS + sex*age.
The total variance explained represents the variance explained by the models described above (from a to f) minus the variance explained by a model with only covariates (dimensional symptoms ∼ age + sex + 10 PCs). Percentage of variance explained was calculated using the Bosker/Snijders pseudo R 2 value (Snijders & Bosker, Reference Snijders and Bosker1994).
Mixed-effects linear regression models confirmed the main effect of ADHD-PRS on CBCL/ABCL Attention Problems, and on SDQ Hyperactivity (Table 2). Similarly, we observed a significant main effect of ERS on CBCL/ABCL Attention Problems, and on SDQ Hyperactivity. Moreover, mixed-effects logistic regression models confirmed that increased ADHD-PRS was associated with higher odds of ADHD diagnosis (Table S11). Similarly, higher odds of ADHD diagnosis were observed with increased ERS. Although an interactive effect between ADHD-PRS and ERS did not reach statistical significance for the ADHD categorical model, it was marginally significant (Table S11). Sensitivity analyses with additional PRS thresholds were performed, and they can be found in Tables S12 and S13.
Depressive and anxiety disorders
Mixed-effects linear regression models demonstrated a significant main effect of ERS on CBCL/ABCL Depression/Anxiety and on SDQ Emotion (Table 2). We observed a statistically significant main effect of ADHD-PRS on CBCL/ABCL Depression/Anxiety, but not on SDQ Emotion. The interaction between ERS and ADHD-PRS did not reach statistical significance neither for CBCL/ABCL Depression/Anxiety nor for SDQ Emotion (Table 2).
Mixed-effects logistic regression models demonstrated a significant main effect of ERS on the diagnosis of depressive disorders and on the diagnosis of anxiety disorders (Table S11). However, the main effect of ADHD-PRS did not reach statistical significance for the diagnosis of depressive disorders or anxiety disorders. Similarly, the interaction between ADHD-PRS and ERS did not reach statistical significance for the diagnosis of depressive disorders or anxiety disorders (Table S11). Sensitivity analyses with additional PRS thresholds were performed, and they can be found in Tables S14, S15, and S16.
Effects of age
Mixed-effects linear regression models demonstrated a significant interaction between ERS and age on CBCL/ABCL Attention Problems (Table 2), indicating a decreased effect of ERS with increased age (Figure 2). More specifically, marginal effect analysis revealed that older age was associated with lower ADHD symptoms for individuals with ERS z-score > −1. The strength of the association increased with increasing ERS. For example, at ERS of −1 z-score, there was a yearly decrease of .02 z-score on CBCL/ABCL Attention Problems (95% CI −.02, −.01; p < .0001). At ERS of 1 z-score, the yearly decrease was of .04 (95% CI .05, .03; p < .0001), and at ERS of 2 z-scores, the yearly decrease was of .05 (95% CI −.06, .03; p < .0001).
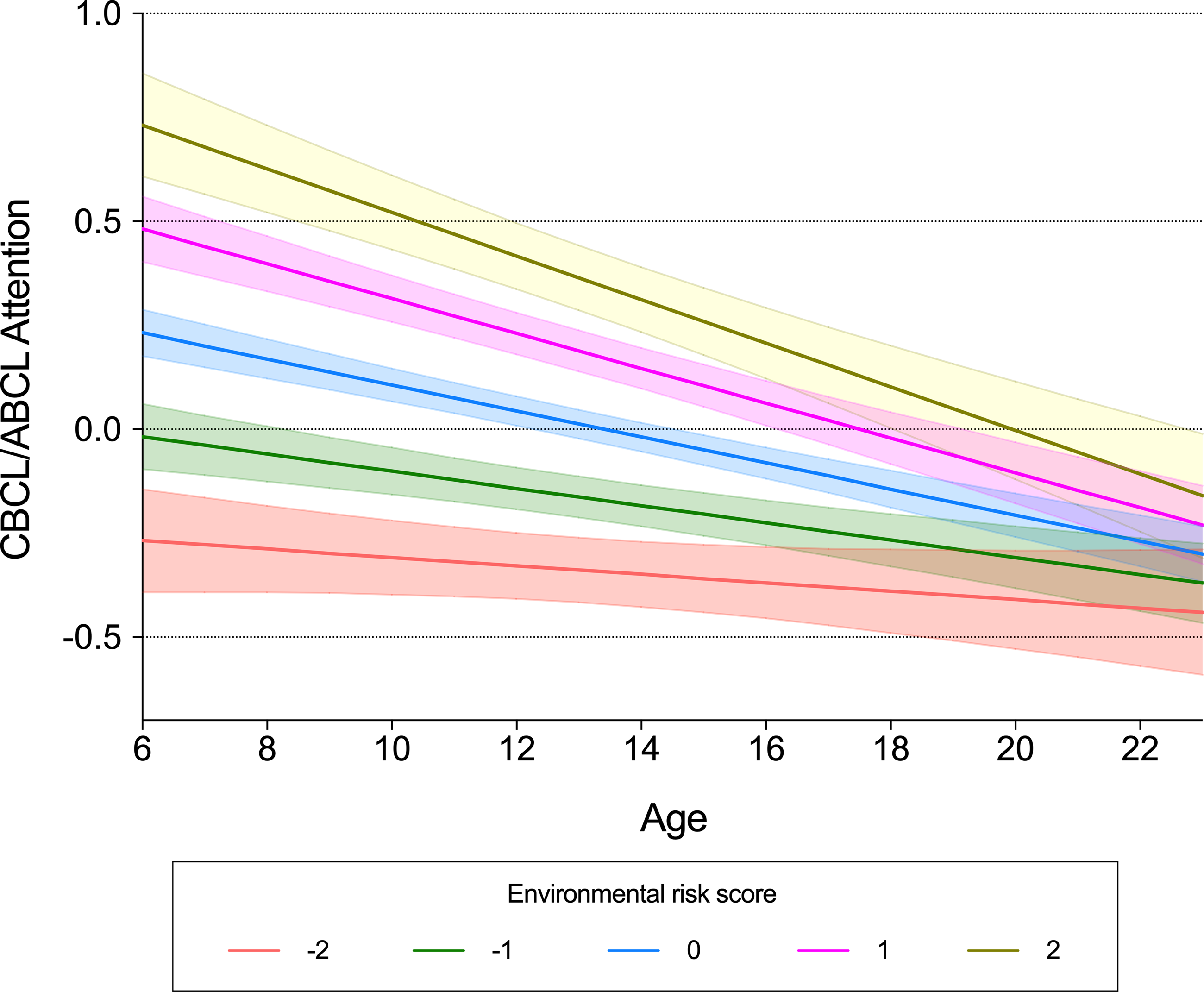
Figure 2. Interactive effects between environmental risk scores and age on symptoms of attention-deficit/hyperactivity disorder. Mean predicted values and 95% confidence intervals of dimensional symptoms of ADHD estimated from linear mixed-effects models testing the interaction between ERS and age. Data for CBCL/ABCL Attention and ERS are presented as z-scores. CBCL = Child Behavior Checklist; ABCL = Adolescent Behavior Checklist; SDQ = Strength and Difficulties Questionnaire; ERSs = environmental risk score; ADHD = attention-deficit/hyperactivity disorder. Model = Dimensional symptoms ∼ ERS*age + ERS + age + sex + sex*ERS + sex*age.
No significant interaction between ERS and age was observed on SDQ Hyperactivity, CBCL/ABCL Depression/Anxiety, or SDQ Emotion (Table 2). No significant interaction was observed between ADHD-PRS and age on dimensional psychopathology measures (Table 2). Mixed-effects logistic regression models did not reveal statistically significant interactions between ERS and age, or between ADHD-PRS and age, on the diagnosis of ADHD, depressive disorders, or anxiety disorders (Table S11).
Discussion
GxE studies have an indisputable impact on psychiatric research. However, partially due to the candidate gene and candidate environment approach, they suffer from low statistical power and replication rates. In this sense, the use of combined scores to represent the liability conferred by genetic and environmental factors could be a valuable approach. ADHD is a suitable candidate for studying GxE in psychiatry for presenting high heritability and well-defined environmental risk factors. Our findings demonstrate that environmental and genetic factors act synergistically in the development of ADHD symptoms in children and adolescents. More specifically, the strength of the association between symptoms of ADHD and ADHD-PRS increased as a function of growing ERS, and individuals with a high genetic risk are more likely to be negatively affected by the environmental risks. Thys type of GxE is known as diathesis-stress model (Monroe & Simons, Reference Monroe and Simons1991). On the other hand, no effect of ADHD-PRS was observed among subjects with low exposure to environmental factors. Furthermore, while our results suggest that environmental risk factors for ADHD are also associated with symptoms of depression and anxiety, their interaction with PRSs appears to be specific for symptoms of ADHD. As far as we are aware, this is the first study to evaluate GxE in ADHD using multivariable combined scores to represent both the liability conferred by genetic and environmental factors.
In this study, we showed a significant interaction between genetic and environmental factors in the development of symptoms of ADHD in children and adolescents, using a combined risk score to represent each one. The notion that environmental stressors could predispose to the development of psychiatric disorders in individuals possessing innate biological predispositions has been debated for centuries (Kendler, Reference Kendler2020). ADHD is a promising disorder to study GxE due to its high heritability of 70–80% estimated from twin studies (Faraone & Larsson, Reference Faraone and Larsson2019). The use of PRS is particularly relevant for ADHD since the disorder has a highly polygenic genetic architecture, with common variants playing an important role (Demontis et al., Reference Demontis, Walters, Martin, Mattheisen, Als, Agerbo, Baldursson, Belliveau, Bybjerg-Grauholm, Bækvad-Hansen, Cerrato, Chambert, Churchhouse, Dumont, Eriksson, Gandal, Goldstein, Grasby and Grove2019). The studies of Østergaard et al., (Østergaard et al., Reference Østergaard, Trabjerg, Als, Climent, Privé, Vilhjálmsson, Bækvad-Hansen, Bybjerg-Grauholm, Hougaard, Nordentoft, Werge, Demontis, Mortensen, Børglum, Mors and Agerbo2020) and Stojanovski et al., (Stojanovski et al., Reference Stojanovski, Felsky, Viviano, Shahab, Bangali, Burton, Devenyi, O’Donnell, Szatmari, Chakravarty, Ameis, Schachar, Voineskos and Wheeler2018), however, are exceptions of the candidate gene approach. Østergaard et al., (Østergaard et al., Reference Østergaard, Trabjerg, Als, Climent, Privé, Vilhjálmsson, Bækvad-Hansen, Bybjerg-Grauholm, Hougaard, Nordentoft, Werge, Demontis, Mortensen, Børglum, Mors and Agerbo2020) investigated the interaction between ADHD-PRS and psychosocial risk factors and found no significant synergistic effect. On the other hand, Stojanovski et al., (Stojanovski et al., Reference Stojanovski, Felsky, Viviano, Shahab, Bangali, Burton, Devenyi, O’Donnell, Szatmari, Chakravarty, Ameis, Schachar, Voineskos and Wheeler2018) observed a significant interaction between ADHD-PRS and mild traumatic brain injury (TBI). Interestingly, the authors observed that ADHD-PRS were associated with ADHD symptoms only in subjects with no TBI, suggesting a reduced role of genetics in subjects exposed to mild trauma (Stojanovski et al., Reference Stojanovski, Felsky, Viviano, Shahab, Bangali, Burton, Devenyi, O’Donnell, Szatmari, Chakravarty, Ameis, Schachar, Voineskos and Wheeler2018). These findings point to an opposite direction when compared to our study and could indicate a distinct GxE mechanism for TBI. However, the largest meta-analysis assessing the association between ADHD and TBI showed that severe TBI, but not mild or moderate, was a risk factor for ADHD (Asarnow et al., Reference Asarnow, Newman, Weiss and Su2021). On the other hand, across TBI injury severity, the rate of preinjury ADHD diagnoses was significantly higher than the general population (Asarnow et al., Reference Asarnow, Newman, Weiss and Su2021). These findings suggest that mild and moderate TBI are not risk factors for ADHD, but more likely a consequence of symptoms from the disorder.
According to our findings, children exposed to environmental risk factors for ADHD are more likely to develop inattention and hyperactivity/impulsivity symptoms. On the other hand, a protective environment can act as a buffer against the genetic liability for ADHD. Most risk factors included in the ERS exert their effects in the prenatal and perinatal periods (Kim et al., Reference Kim, Kim, Lee, Jeong, Lee, Lee, Lee, Kronbichler, Stubbs, Solmi, Koyanagi, Hong, Dragioti, Jacob, Brunoni, Carvalho, Radua, Thompson, Smith and Fusar-Poli2020), which are critical moments of brain development (VanRyzin et al., Reference VanRyzin, Pickett and McCarthy2018). The pathophysiological mechanisms underlying environmental risk factors for ADHD are highly heterogeneous and have been hypothesized to comprise: (1) toxic effects of chemical products due to maternal smoking (Huang et al., Reference Huang, Wang, Zhang, Zheng, Zhu, Qu and Mu2018); (2) hypoxia in the developing fetal brain due to hypertensive disorders of pregnancy (Maher et al., Reference Maher, O’Keeffe, Kearney, Kenny, Dinan, Mattsson and Khashan2018) and maternal DM (Zeng et al., Reference Zeng, Tang, Yue, Li, Qiu, Hu, Tang, Wang, Yang, Qu and Mu2019); (3) long-term dysregulation of fetal hypothalamic–pituitary–adrenal axis due to maternal stress (Manzari et al., Reference Manzari, Matvienko-Sikar, Baldoni, O’Keeffe and Khashan2019); (4) changes in the microbiota of offspring with consequent impaired immune development from cesarean delivery (Polidano et al., Reference Polidano, Zhu and Bornstein2017; Zhang et al., Reference Zhang, Sidorchuk, Sevilla-Cermeno, Vilaplana-Perez, Chang, Larsson, Mataix-Cols and Fernandez de la Cruz2019); (5) brain immaturity with decreased resilience to external insults from preterm birth (Allotey et al., Reference Allotey, Zamora, Cheong-See, Kalidindi, Arroyo-Manzano, Asztalos, van der Post, Mol, Moore, Birtles, Khan and Thangaratinam2018; Franz et al., Reference Franz, Bolat, Bolat, Matijasevich, Santos, Silveira, Procianoy, Rohde and Moreira-Maia2018); (6) absence of neuroprotective effects of breast milk in children who were never breastfed (Zeng et al., Reference Zeng, Tang, Tang, Shi, Zhang, Zhu, Xiao, Qu and Mu2020); and (7) disruption in the development of neurotransmitter systems from antidepressant use during pregnancy (Jiang et al., Reference Jiang, Peng, Zhang and Ruan2018; Leshem et al., Reference Leshem, Bar-Oz, Diav-Citrin, Gbaly, Soliman, Renoux and Matok2021). Furthermore, low maternal education, low maternal age, and single-parent family are supposed to represent a more complex set of risk factors related to social vulnerability (Russell et al., Reference Russell, Ford, Williams and Russell2016). Similar to the environmental influences, the mechanisms behind the genetic liability for ADHD are related to impaired neurodevelopmental processes, including synapse formation, neuronal plasticity, and neurotransmitter homeostasis (Demontis et al., Reference Demontis, Walters, Martin, Mattheisen, Als, Agerbo, Baldursson, Belliveau, Bybjerg-Grauholm, Bækvad-Hansen, Cerrato, Chambert, Churchhouse, Dumont, Eriksson, Gandal, Goldstein, Grasby and Grove2019).
Our findings show that environmental risk factors for ADHD are associated with increased symptoms of depression and anxiety in the population and with higher rates of depressive and anxiety disorders diagnosed according to DSM-IV. These results suggest a broad impact of environmental stressors in the susceptibility to psychopathology that is not specific to ADHD. These findings are in accordance with previous studies highlighting the transdiagnostic effect of environmental risk factors (Arango et al., Reference Arango, Dragioti, Solmi, Cortese, Domschke, Murray, Jones, Uher, Carvalho, Reichenberg, Shin, Andreassen, Correll and Fusar-Poli2021). Moreover, prior results demonstrate that environmental risk factors are associated with psychiatric comorbidities in patients with ADHD (Owens & Hinshaw, Reference Owens and Hinshaw2013). We did not observe a consistent increased risk for a diagnosis of depressive disorders or anxiety disorders in individuals with higher ADHD-PRS. However, we observed that ADHD-PRSs were associated with higher symptoms of depression and anxiety in the whole population. These findings are consistent with prior studies demonstrating an association between ADHD-PRS and depression or anxiety (Martin et al., Reference Martin, Taylor, Rydell, Riglin, Eyre, Lu, Lundström, Larsson, Thapar and Lichtenstein2018; Rice et al., Reference Rice, Riglin, Thapar, Heron, Anney, O’Donovan and Thapar2019). Furthermore, they support previous studies showing that ADHD-PRSs have a broad influence on childhood psychopathology (Brikell et al., Reference Brikell, Larsson, Lu, Pettersson, Chen, Kuja-Halkola, Karlsson, Lahey, Lichtenstein and Martin2018). The relationship between genetic risk for ADHD and symptoms of depression/anxiety is also in accordance with studies demonstrating a high genetic overlap between distinct psychiatric disorders, with several contributing loci playing a pleiotropic role (“Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders.,” Reference Lee, Anttila, Won, Feng, Rosenthal, Zhu, Tucker-Drob, Nivard, Grotzinger, Posthuma, Wang, Yu, Stahl, Walters, Anney, Duncan, Ge, Adolfsson, Banaschewski and Belangero2019). Although a genetic overlap between psychiatric disorders has been demonstrated in previous studies, our findings highlight that environmental risk factors are likely more relevant in the association between ADHD and depression/anxiety. Future studies should be designed to disentangle the role of environment and genetics in psychiatric comorbidities in ADHD.
Our results should be viewed in light of some limitations. First, it is important to stress that even though the literature on the impact of environmental factors on the risk for ADHD is broad, the identification of causal associations is still a challenge, and we cannot rule out that some of the effects observed are due to unknown confounding and reverse causation. For example, smoking during pregnancy could be related to ADHD in the offspring due to genetic confounders (Obel et al., Reference Obel, Zhu, Olsen, Breining, Li, Grønborg, Gissler and Rutter2016). The association between maternal use of antidepressants and ADHD has also been suggested to result from confounding since this effect has been shown to persist in children whose mothers were exposed to antidepressants only before pregnancy (Leshem et al., Reference Leshem, Bar-Oz, Diav-Citrin, Gbaly, Soliman, Renoux and Matok2021). Furthermore, rates of ADHD in children whose mothers used antidepressants were similar to the rates of ADHD in children whose mothers had untreated psychiatric conditions, suggesting that maternal psychiatric disorder might be a bias in the association (Jiang et al., Reference Jiang, Peng, Zhang and Ruan2018). Even though our analyses indicate the absence of significant gene–environment correlations, we could not explore possible confounding effects of environment–environment interactions. We only included environmental risk factors available in our dataset for which associations with ADHD have been summarized in meta-analyses. Although scientifically consistent, this methodological approach likely excluded additional relevant risk factors. Finally, although the ADHD-PRS and ERS may be useful for prediction of symptoms and diagnosis, they do not inform GxE mechanistic process directly.
This is the first study to demonstrate an interactive effect between genetic and environmental factors in ADHD using a combined score to represent each one. Put simply, the synergistic effect indicates that the combined effect between gene and environment is more significant than the sum of each individually. Mechanistically, our findings suggest that environmental stressors modulate the genetic risk for ADHD. Clinically, our results highlight that it might make sense to investigate if reducing environmental risks could be a potentially effective measure to prevent the development of symptoms of ADHD, especially in children with a family history of the disorder. Finally, the use of multivariable combined scores in GxE studies could be useful to overcome some of the limitations intrinsic to the candidate gene and candidate environment approach.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579423000366
Acknowledgements
This study was supported by the National Institute of Developmental Psychiatry for Children and Adolescents, São Paulo, with grants from the São Paulo Research Foundation (Fapesp 2014/50917-0; 2013/08531-5) and the Brazilian National Council for Scientific and Technological Development (CNPq 465550/2014-2). DTL was supported by a CNPq postdoctoral fellowship (grant number 154116/2018-1) and supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (grant number 29486). We thank Mauricio Scopel Hoffmann, MD, PhD, Universidade Federal de Santa Maria, Santa Maria, Brazil, for the help with the inverse probability weights.
Conflicts of interest
LAR has received grant or research support from, served as a consultant to, and served on the speakers’ bureau of Aché, Bial, Medice, Novartis/Sandoz, Pfizer/Upjohn, and Shire/Takeda in the last three years. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by Dr LAR have received unrestricted educational and research support from the following pharmaceutical companies in the last three years: Novartis/Sandoz and Shire/Takeda. Dr LAR has received authorship royalties from Oxford Press and ArtMed. All other authors report no financial relationships with commercial interests.







