LINKS BETWEEN SEAFOOD AND HUMAN HEALTH AND WELL-BEING
Marine living resources provide an excellent example of how human activities and the ocean environments are inseparably linked, having both benefits and risks (Bowen et al., Reference Bowen, Halvarson and Depledge2006; Brunner et al., Reference Brunner, Jones, Friel and Bartley2009; Tacon & Metian, Reference Tacon and Metian2013). In this review we describe the complex links that exist between seafood and human health and well-being.
Seafood's role in food security in coastal developing countries
Seafood (particularly fish) plays an important role in food security as a key source of food and nutrition for coastal regions of developing countries, contributing almost a quarter of the animal protein consumed by people in Low Income Food Deficit Countries (FAO, 2014; United Nations, 2014). Fish is the main source of animal protein along with essential micronutrients and fatty acids for 3 billion people (United Nations, 2014). Populations in Africa and Asia rely even more on fish for their intake of animal proteins, and this contribution can reach up to 40% or more in some small developing island states (United Nations, 2014). Overall, it is estimated that fish contributes about 17% to the world's animal protein intake and that about 400 million people get more than 50% of their animal protein from fish (FAO, 2014; United Nations, 2014). There is concern that the availability of seafood may not be able to keep up with demand, considering that worldwide demand for seafood products has increased from about 10 kg per person each year during the 1960s to 19.0 kg per person in 2010–2012, and that the world's population is expected to grow by 20% from 2010 to 2030 (FAO, 2014). Given a current fisheries and aquaculture production for a human consumption estimated to be about 136 million tonnes (animals from capture fisheries and aquaculture), with annual per capita fish consumption remaining at about 19 kg per person, a similar proportion of fish going into fishmeal, fish oil and other non-food uses as today, and a world population of 9.6 billion people, approximately 47.5 million additional tonnes of food fish will be needed in 2050 (FAO, 2014).
Although fish plays an extremely important role in the supply of protein in many developing coastal countries, it has sometimes been considered more important as a source of micronutrients as more than 2 billion people, especially in developing countries, are undernourished due to a lack of essential vitamins and minerals, often contained in seafood (FAO, 2014). Important micronutrients provided by fish consumption includes certain vitamins, the essential amino acid lysine (Médale et al., Reference Médale, Lefèvre and Corraze2003) together with minerals such as selenium, an essential dietary trace element that plays an important role in antioxidant defence systems and may protect against cardiovascular disease and the toxic effects of mercury (Mozaffarian, Reference Mozaffarian2009). Furthermore, seafood species are particularly rich in iron, and its deficiency is considered the most common single-nutrient deficiency syndrome in the world affecting haemoglobin counts most seriously in women, children and adolescents from developing countries (Trowbridge & Martorell, Reference Trowbridge and Martorell2002; Bagchi, Reference Bagchi2004).
The importance of seafood in the developed world
In the developed world (i.e. Europe and the USA), seafood does not play such an important role in food security, since people usually rely on animal protein from other sources (e.g. livestock). Most people in developed countries get sufficient protein in their diet and therefore much attention has been given to the contribution of seafood to a healthy diet because of the health benefits provided by the long-chain omega-3 (or n-3) fatty acids contained in seafood (reviewed by Brunner et al., Reference Brunner, Jones, Friel and Bartley2009; Lloret, Reference Lloret2010). First, omega-3 fatty acids from fish oil help to improve cardiovascular health by decreasing risk factors such as triglyceride concentrations, blood pressure, platelet aggregation and heart arrhythmias, thus reducing coronary heart disease mortality (see e.g. He et al., Reference He, Song, Daviglus, Liu, Van Horn, Dyer and Greenland2004; Mozaffarian & Rimm, Reference Mozaffarian and Rimm2006). Second, fish-derived omega-3 fatty acids consumption protects against the development of certain cancers, e.g. breast and prostate cancers (see e.g. Fernandez et al., Reference Fernandez, Chatenoud, La Vecchia, Negri and Franceschi1999). Cardiovascular diseases and cancer are amongst the most important causes of ill-health in developed countries according to the World Health Organization (2009a, b) and in this regard, the specific roles of a-linolenic acid (ALA, 18:3n-3), eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) in preventing cardiovascular disease and cancer are subjects of active research (see e.g. He et al., Reference He, Song, Daviglus, Liu, Van Horn, Dyer and Greenland2004; Mozaffarian & Rimm, Reference Mozaffarian and Rimm2006).
In addition to the cardiovascular and cancer protective effects of fish consumption, fish intake has been also associated with other health outcomes, such as reduced depression symptoms in adults and fewer asthmatic and respiratory allergies in children (reviewed by Lloret, Reference Lloret2010). Omega-3 fatty acids also mediate the inflammatory process and influence the general health status of the skeletal system (reviewed by Brunner et al., Reference Brunner, Jones, Friel and Bartley2009). The omega-3 fatty acids found in seafood are an important component of the diet in some regions where people display better than average health outcomes. Thus for example, in Mediterranean countries, the traditional diet (so-called ‘Mediterranean diet’), rich in seafood, has consistently been shown to be associated with favourable health outcomes and a better quality of life (reviewed by Sofi et al., Reference Sofi, Abbate, Gensini and Casini2008; Sofi, Reference Sofi2009). Several epidemiological and observational studies suggest that the type of diet traditionally followed by Mediterranean people may protect against chronic diseases and mortality, with Mediterranean nations presenting lower rates of cardiovascular disease and cancer in comparison with other nations (Trichopoulou, Reference Trichopoulou2001; Benetou et al., Reference Benetou, Trichopoulou, Orfanos, Naska, Lagiou, Boffetta and Trichopoulos2008). Greater adherence to this diet has also been associated with longevity (Trichopoulou, Reference Trichopoulou2004), a reduction in depressive disorders (Sánchez-Villegas et al., Reference Sánchez-Villegas, Delgado-Rodríguez, Alonso, Schlatter, Lahortiga, Serra Majem and Martínez-González2009) and alleviation of iron deficiency (Mesías et al., Reference Mesías, Seiquer, Muñoz-Hoyos, Galdó and Navarro2009).
In particular, pelagic fish such as sardines (Sardina spp.), anchovies (Engraulis spp.), mackerels (Scomber spp.) and tunas (Thunnus spp.) represent a good dietary source of omega-3 fatty acids (reviewed by Lloret et al., Reference Lloret, Shulman and Love2014). The edible muscle of these pelagic fishes can contain up to 10 times more total lipids and omega-3 fatty acids than that of demersal fish species. This is because pelagic species tend to concentrate the lipid reserves in their muscle whereas demersal species tend to concentrate lipids in their liver or mesenteries, which usually are not consumed (Lloret et al., Reference Lloret, Shulman and Love2014). The key role of pelagic species in global omega-3 supply is more apparent if we consider that they comprise the largest proportion of the global marine catches: small pelagics (herrings, sardines, anchovies, etc.) contributed about 22% (19.9 million tonnes) of the total catch in 2009 (this share is down from 29% in the 1950s and 27% in 1970s), whereas the large pelagics (e.g. tuna, bonito, billfish etc.) accounted for 19% (16.6 million tonnes) of total catches in 2009 (FAO, 2011). Although marine capture fisheries have always been the largest contributor to world fish production, in the last two decades marine and inland aquaculture has expanded rapidly, and the relative contribution of marine capture fisheries to the growing total world fish production has fallen (FAO, 2014): in 1950, marine capture was 16.7 million tonnes and accounted for 86% of total world fish production whereas in 2009 marine capture contributed 49% of the world's fish production in comparison with mariculture (21%), freshwater aquaculture (23%) and inland capture fishery (6%).
Balancing benefits and risks linked to eating seafood
Despite the potential human benefits derived from seafood consumption, pathogens, biotoxins and chemical contamination are threatening seafood quality and quantity throughout the world, thus seriously impacting the nutritional value of seafood and posing risks to human health and well-being (reviewed by Ross & Birnbaum, Reference Ross and Birnbaum2003; Fleming et al., Reference Fleming, Broad, Clement, Dewailly, Elmir, Knap, Pomponi, Smith, Solo Gabriele and Walsh2006; Lloret, Reference Lloret2010). Pathogens including parasites, pollutants such as heavy metals, persistent organic pollutants (POPs) and biotoxins are affecting the safety of seafood. Although seafood safety concerns both the developing and the developed world, it is in the latter where most discussion and studies are focused. In Europe and the USA, the health benefits and risks associated with seafood consumption are leading to controversy. For example, concerns regarding potential health risks of polychlorinated biphenyls/dioxins and mercury, which are present in some fish species (particularly in large pelagic fish, which are also the richest in omega-3 fatty acids), have tempered the perception of fish as a healthy food (Mozaffarian & Rimm, Reference Mozaffarian and Rimm2006; Mozaffarian, Reference Mozaffarian2009). Methylmercury, the predominant form of mercury in fish, can accumulate to high concentrations in predatory fish such as tunas and swordfish because once it enters the food web, it increases in concentration with each successive trophic level, posing a health risk to humans who eat these predatory fish (Drevnick et al., Reference Drevnick, Lamborg and Horgan2015). For developing foetuses, infants and children, the primary health impact of methylmercury is impaired neurological development (Rice et al., Reference Rice, Swartout, Mahaffey and Schoeny2000) whereas for adults, methylmercury exposure has been associated with increased rates of cardiovascular disease (Guallar et al., Reference Guallar, Sanz-Gallardo, Van't Veer, Bode, Aro, Gómez-Aracena, Kark, Riemersma, Martín-Moreno and Kok2002). It is also important to consider that, on average, seafood having greater ecological impact (i.e. captured species that show the highest vulnerability to fishing pressures) also present higher health risks (as indexed by mercury concentration) and do not necessarily provide higher health benefits (as indexed by omega-3 fatty acid concentrations; Gerber et al., Reference Gerber, Karimi and Fitzgerald2012).
Another factor that is tempering the perception of fish as a healthy food is the presence of parasites because some fish parasites (e.g. Anisakids) can cause digestive disorders and/or allergies in humans as a consequence of accidental ingestion of raw, undercooked or improperly processed fish and/or cephalopods parasitized by larvae of these parasites (Valero et al., Reference Valero, Terrados, Díaz, Reguera and Lozano2003; Audícana & Kennedy, Reference Audícana and Kennedy2008). Parasites can also impinge fisheries, reducing the marketability of harvested products and reducing both abundance and yield by increasing mortality, reducing fish condition (lipids) as well as fecundity and egg quality (Dobson & May, Reference Dobson and May1987; Lloret et al., Reference Lloret, Muñoz and Casadevall2012; Gómez & Nichols, Reference Gómez and Nichols2013; Ferrer-Maza et al., Reference Ferrer-Maza, Lloret, Muñoz, Faliex, Vila and Sasal2014, Reference Ferrer-Maza, Muñoz, Lloret, Faliex, Vila and Sasal2015).
Currently, the links between fish and human health are changing due to many factors, amongst which (over-) fishing, aquaculture and climate change are the most important. Because changes in fish condition and health will ultimately affect the health of consumers, it is important to consider how global change affects the condition and productivity of fish. The following sections attempt to summarize how the complex links between marine living resources and human health and well-being are being challenged by fisheries, aquaculture and climate change, and provides suggestions to promote fish security and safety in the context of global change.
FISHING AND AQUACULTURE: IMPACTS ON FISH AND HUMAN HEALTH
Capture fisheries
Fishing is threatening a number of fish stocks that have increasingly become over-exploited: the number of over-exploited marine fish stocks has increased during recent decades, from 10% in 1970 to 29% in 2011, while a further 61% of fish stocks are currently assessed as sustainably (fully) exploited (FAO, 2014). Over-exploitation, particularly of pelagic oily fish, is significantly reducing the overall supply of long chain omega-3 fats and proteins. For the developed world, the collapse of fisheries due to reduced fish productivity does not pose a significant risk to general food security, but more to health, because there is a danger that there will not be enough omega-3 from fish to assure optimum health support for all. The current recommendations of governmental health agencies to people in developed countries, to increase their intake of fatty fish by at least 2–3-fold, are inconsistent with the stagnation of global production of capture fisheries (Jenkins et al., Reference Jenkins, Sievenpiper, Pauly, Sumaila, Kendall and Mowat2009). In contrast, for many coastal countries of the developing world, the collapse of fish production poses a real risk for food security. Furthermore, overfishing is decreasing the biological and genetic diversity of fish worldwide, adversely impacting seafood production (Malin & Palumbi, Reference Malin and Palumbi2014). Decreased diversity in marine ecosystems may also increase the risk of pathogen emergence that can pose a threat to consumer health, as it occurs in land, where decreased diversity in agroecosystems increases the risk of pest attack (Swift et al., Reference Swift, Izac and van Noordwijk2004). Overfishing particularly affects the health of the poorest people because it undermines food security in the coastal regions of less economically developed countries, where overfishing reduces the supply of a vital source of dietary protein (Brunner et al., Reference Brunner, Jones, Friel and Bartley2009). This inequality persists within rich economies, where the decreasing affordability of fish in the diet, likewise favours the better off (Brunner et al., Reference Brunner, Jones, Friel and Bartley2009).
Fishing can further reduce the supply of omega-3 and proteins indirectly. First, by reducing the food supply of exploited fish where a reduced quality and quantity of prey translates into lower fish condition (Lloret et al., Reference Lloret, Shulman and Love2014). For example, trawling affects biomass and production of benthic communities (Jennings et al., Reference Jennings, Dinmore, Duplisea, Warr and Lancaster2001) that are the main food source for a number of exploited demersal species (Hoines & Bergstad, Reference Hoines and Bergstad1999). As a result, bottom trawling has the potential to affect the condition of exploited species, e.g. red mullet (Mullus barbatus) in the north-western Mediterranean (Lloret et al., Reference Lloret, Demestre and Sanchez-Pardo2007) and haddock (Melanogrammus aeglefinus) in the North Sea (Hiddink et al., Reference Hiddink, Jennings and Kaiser2005). Second, the effect of ‘selective fisheries’, i.e. fisheries that for commercial reasons mainly target certain fish sizes is also an issue. This problem is typical of some coastal small-scale (artisanal) and recreational fisheries which, through the selective removal of the largest individuals, affect the lipid reserves and viability of many coastal fish populations because larger individuals are usually better conditioned than the smaller ones (Lloret et al., Reference Lloret, Zaragoza, Caballero, Casadevall and Riera2008, Reference Lloret, Muñoz and Casadevall2012). Third, fishing-induced stress may also affect the condition of marine exploited species. For example, dredging chronically affects the condition of the clam Spisula solida in Portuguese waters, where dredging-induced stress provokes a decrease in lipid composition (Chícharo et al., Reference Chícharo, Chícharo, Gaspar, Regala and Alves2002). Also, noise from fishing operations (e.g. from towed nets or from motors) may induce stress in fish and reduce condition (Anderson et al., Reference Anderson, Berzins, Fogartya, Hamlin and Guillette2011).
Fishing can also exert important effects on both marine community structure and ecosystem functioning via reductions in the abundance and/or diversity of parasites, many of which have a substantial ecological role in marine ecosystems (Wood et al., Reference Wood, Micheli, Fernández, Gelcich, Castilla and Carvajal2013). Parasites are extremely diverse, have key roles in ecological and evolutionary processes, and infection may paradoxically result in ecosystem services of direct human relevance (Gómez et al., Reference Gómez, Nichols, Perkins, Aguirre, Ostfeld and Daszak2012; Gómez & Nichols, Reference Gómez and Nichols2013). Fishing can drive declines in overall parasite species richness and parasite abundance by reducing the availability of habitat and resources for parasites, probably because trophically transmitted parasites require multiple host species, some of which are the top predators most sensitive to fishing impacts (Wood et al., Reference Wood, Micheli, Fernández, Gelcich, Castilla and Carvajal2013). Nevertheless, the response of parasites to fishing is variable and context specific, with parasite responses being mediated by parasite traits and the host's response to fishing in a particular area (Wood et al., Reference Wood, Sandin, Zgliczynski, Guerra and Micheli2014). This is an aspect of parasites that contrasts to the effects of fish parasites on human health and well-being noted earlier.
Marine protected areas
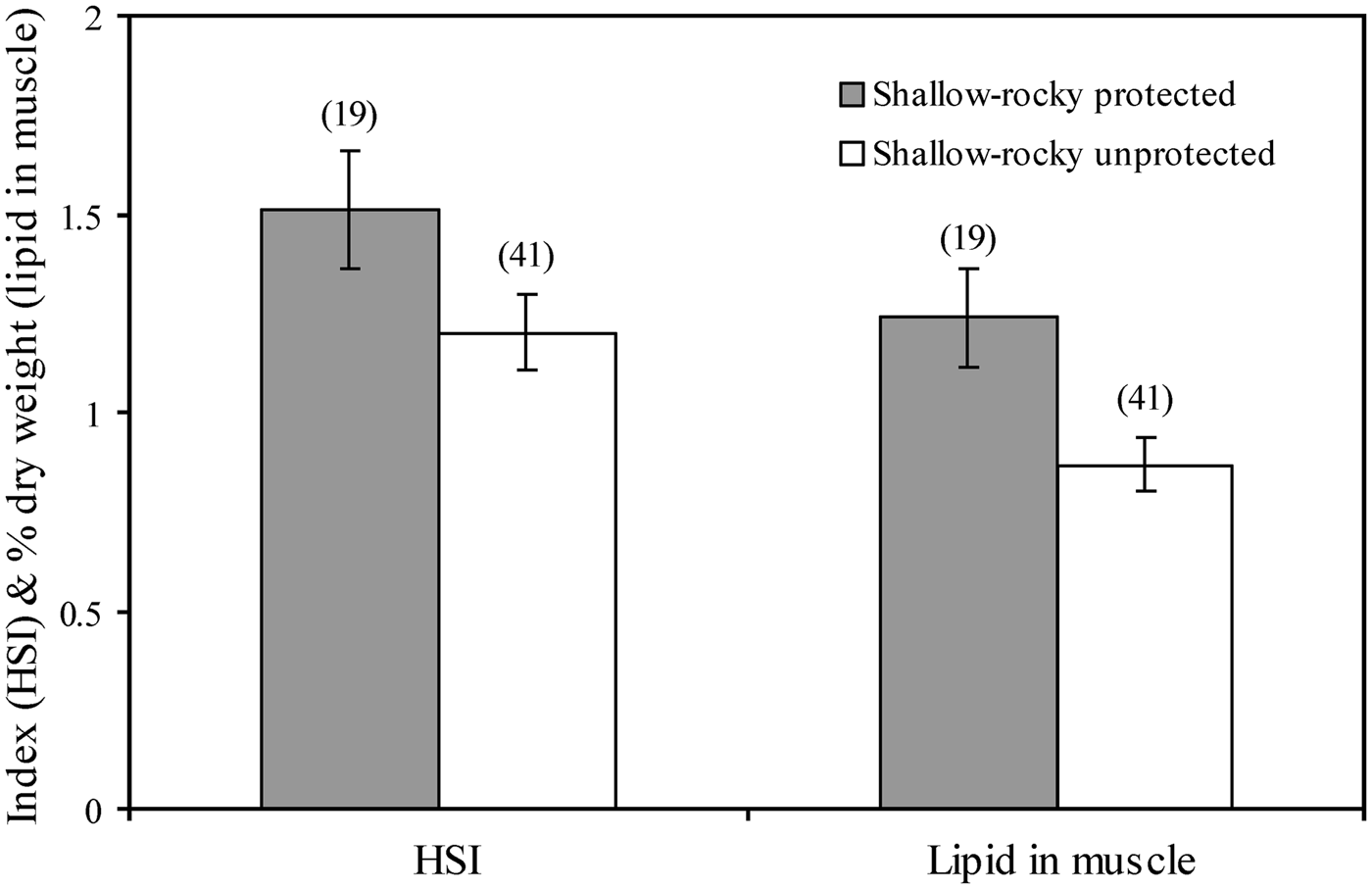
Marine protected areas, where fishing is restricted to varying degrees, have the potential to enhance not only fish abundance and biomass (Planes et al., Reference Planes, Galzin, Garcia Rubies, Goni, Harmelin, Le Direach, Lenfant and Quetglas2000), but also the condition of fish species inhabiting these areas, and can be regarded therefore as a tool to enhance fish oil reserves and human health. For example, white seabream (Diplodus sargus) is better conditioned (higher lipid content in the muscle and higher hepatosomatic index, a proxy of fat reserves stored in the liver) within the rocky areas of two marine reserves in the north-western Mediterranean than in adjacent unprotected rocky areas (Figure 1; Lloret & Planes, Reference Lloret and Planes2003). These examples show that marine reserves not only contribute to preserve biodiversity and enhance fish abundance and biomass, but also support increased lipid reserves in exploited species, providing long-term benefits to local fisheries and consumer's health.

Fig. 1. Differences in hepatosomatic index (HSI) and muscle lipid content of post-spawners of white seabream (Diplodus sargus) between rocky protected and rocky unprotected areas (from Lloret & Planes, Reference Lloret and Planes2003).
Furthermore, recent studies have shown that under the influence of certain parasites, fish stocks are less disposed to collapse if marine reserves are present (McCallum et al., Reference McCallum, Gerber and Jani2005). These authors found that the presence of the Rickettsia-like prokaryote does not necessarily decrease yield of its host – the exploited invertebrate species abalone, Haliotis sp. – when a reserve is present. In contrast, when a reserve is absent, this highly transmissible pathogen causes a rapid decline in equilibrium yield for efforts beyond those that produce maximum sustainable yield, making the fishery more prone to collapse (McCallum et al., Reference McCallum, Gerber and Jani2005). Notwithstanding these results, the links between parasites and marine reserves are still not well understood: several studies have found that areas where fishing is prohibited or strictly regulated can strengthen the life cycle of parasites and their trophic links and, consequently, facilitate parasite abundance and/or diversity among fished host species (e.g. Sasal et al., Reference Sasal, Faliex and Morand1996; Bartoli et al., Reference Bartoli, Gibson and Bray2005, Loot et al., Reference Loot, Aldana and Navarrete2005, Hechinger et al., Reference Hechinger, Lafferty and Kuris2008; Wood et al., Reference Wood, Micheli, Fernández, Gelcich, Castilla and Carvajal2013; Aldana et al., Reference Aldana, Pulgar, Orellana, Patricio Ojeda and García-Huidobro2014). Other studies have found lower parasite abundance in marine reserves compared with open access areas (Sonnenholzner et al., Reference Sonnenholzner, Lafferty and Ladah2011); others have failed to find significant differences in abundance and diversity between reserve and open access areas (Ternengo et al., Reference Ternengo, Levron, Mouillot and Marchand2009), while still others have found negative effects of fishing on parasite diversity but variable effects on parasite abundance (Wood et al., Reference Wood, Sandin, Zgliczynski, Guerra and Micheli2014).
Aquaculture
Although aquaculture could also be regarded as a way to enhance fish proteins and lipids available to consumers and hence to promote consumer health, in fact the health benefits from reared fish are still controversial. Although farmed fish have a higher fat content than wild caught fish (because farmed fish feed abundantly; Hamilton et al., Reference Hamilton, Hites, Schwager, Foran, Knuth and Carpenter2005), these cultured fish usually have a lower proportion of omega-3 fatty acids (the class of fish lipids that are most linked to human health) in their lipids compared with wild fish (reviewed by FAO, 2014; Lloret et al., Reference Lloret, Shulman and Love2014). Thus for example, despite the fat percentage in the muscle or farmed red porgy Pagrus pagrus (3.0%) being higher than that in wild fish (0.65%), wild red porgy shows higher levels of omega-3 fats than reared (Rueda et al., Reference Rueda, Lopez, Martinez, Zamora, Divanach and Kentouri1997). Similar to this, cultured Seriola dumerilii specimens present a lower proportion of DHA than wild specimens (Rodríguez-Barreto et al., Reference Rodríguez-Barreto, Jerez, Cejas, Bolaños and Lorenzo2012). The lower omega-3 fats in some farmed marine fish species, compared with their wild relatives, presumably is because of the lack of lipids originating from algae and marine phytoplankton (reviewed by Lloret et al., Reference Lloret, Shulman and Love2014).
Furthermore, although farmed fish can be a relatively good source of healthy n-3 fatty acids, they can contain high concentrations of organochlorine compounds such as PCBs, dioxins, chlorinated pesticides and other hazardous substances to human health such as organic contaminants and antibiotics (Hites et al., Reference Hites, Foran, Carpenter, Hamilton, Knuth and Schwager2004; Hamilton et al., Reference Hamilton, Hites, Schwager, Foran, Knuth and Carpenter2005). Sometimes reared fish have been associated with a higher presence of some of these hazardous substances (compared with wild fish), which reduces the net health benefits derived from the consumption of farmed fish compared with wild fish. For example, concentrations of organochlorine contaminants were found to be significantly higher in farmed Atlantic salmon (Salmo salar) than in wild, raising concerns that consumption of farmed fish may pose health risks that detract from the beneficial effects of fish consumption (Hites et al., Reference Hites, Foran, Carpenter, Hamilton, Knuth and Schwager2004).
In addition, aquaculture activities are often related to environmental problems (Buschmann et al., Reference Buschmann, Riquelme, Hernandez Gonzalez, Varela, Jimenez, Henriquez, Vergara, Guíñez and Filun2006; Duarte et al., Reference Duarte, Holmer, Olsen, Soto, Marbà, Guiu, Black and Karakassis2009) such as: (i) habitat loss, pollution and changes in benthic communities (alteration of seabed fauna and flora communities) associated with the discharge of suspended solids, and nutrient and organic enrichment of waters resulting in build-up of anoxic sediments, (ii) impact of escaped fish on the native fish fauna; (iii) introduced species, pests and diseases (e.g. parasites), and (iv) use of fishmeal and fish oil to feed reared fish. From all these problems, the last two directly affect seafood quality and quantity. In marine ecosystems, most severe fish parasitic infections have been reported in aquaculture, probably related to artificial culture conditions, where fish densities are abnormally high (Rohde & Littlewood, Reference Rohde, Littlewood and Rohde2005). Farm-origin parasites can spread and affect the survival of wild fish populations (Krkosek et al., Reference Krkosek, Lewis, Morton, Frazer and Volpe2006). Furthermore, current production of fishmeal for aquaculture appears to be unsustainable and raises social and environmental justice issues (Brunner et al., Reference Brunner, Jones, Friel and Bartley2009). In order to ensure healthy fish and a final product comparable with their wild counterparts, farmed fish need to receive eicosapentaenoic and docosahexaenoic acids largely through their diets (FAO, 2014). Fish and crustacean mariculture currently depend on the use of feeds derived from wild fisheries to receive eicosapentaenoic and docosahexaenoic acids, taking 20–25 million metric tonnes of fishmeal to produce 30 million metric tonnes of fish and crustaceans (Duarte et al., Reference Duarte, Holmer, Olsen, Soto, Marbà, Guiu, Black and Karakassis2009). It has been estimated that about 85% (~136 million tonnes) of the worldwide fisheries production are for direct human consumption (fresh, frozen and canned), whereas the 15% remaining (~22 million tonnes) is used for fishmeal and fish oil production as animal food including aquaculture (FAO, 2014). The aquaculture sector currently consumes about 75% of global fish-oil production, although this percentage seems to be declining owing to the increasing demand for fish oil for supplements and other food purposes (the demand for fish oil for direct human consumption is increasing at an annual growth rate of 15–20%; FAO, 2014).
POTENTIAL CONSEQUENCES OF CLIMATE CHANGE FOR FISH AND HUMAN HEALTH
Apart from the impacts of fishing and aquaculture, climate change is emerging as a key factor that has considerable implications for human-exploited natural resources worldwide. Climate change is leading to a warming in many of the world's seas and oceans, producing spatial and temporal changes in the diversity and productivity of fish (see e.g. Hollowed et al., Reference Hollowed, Barange, Beamish, Brander, Cochrane, Drinkwater, Foreman, Hare, Holt, Ito and Kim2013; Lloret et al., Reference Lloret, Sabatés, Muñoz, Demestre, Solé, Font, Casadevall, Martín and Gómez2015), with consequences for fish security as well as on the availability of fish oils for consumers. Fish can respond to ocean warming by shifting their latitudinal range (e.g. Perry et al., Reference Perry, Low, Ellis and Reynolds2005) and depth range (e.g. Dulvy et al., Reference Dulvy, Rogers, Jennings, Stelzenmüller, Dye and Skjoldal2008). In the following sections, we discuss how changes in fish communities due to factors linked to climate change such as warming, changes in river runoff and ocean acidification may affect availability of fish oil for humans and seafood safety.
Direct impact of sea warming on fish oils available to consumers
Global warming can benefit warm-water species, allowing their expansion into areas they did not previously occupy (e.g. Sabatés et al., Reference Sabatés, Martín, Lloret and Raya2006; Petitgas et al., Reference Petitgas, Alheit, Peck, Raab, Irigoien, Huret, van der Kooij, Pohlmann, Wagner, Zarraonaindia and Dickey-Collas2012; Lloret et al., Reference Lloret, Sabatés, Muñoz, Demestre, Solé, Font, Casadevall, Martín and Gómez2015). The opening of the Suez Canal in the 19th century resulted in the migration of more than 600 tropical Indo-Pacific species into the Mediterranean Sea, and constitutes a sound example of how changes in fish communities may have large ecological and economic impacts (Galil, Reference Galil2008; Lejeusne et al., Reference Lejeusne, Chevalonne, Pergent-Martini, Boudouresque and Pérez2009). The introduction or increase of warm-water (thermophilic) species in response to warming may provide new inputs of fish oil to consumers (local citizens will be able to access new fish oil sources). For example, the increase in abundance and expansion of thermophilic pelagic species such as Sardinella aurita, Trachinotus ovatus, Pomatomus saltatrix and Euthynnus alletteratus in the north-western Mediterranean (Lloret et al., Reference Lloret, Sabatés, Muñoz, Demestre, Solé, Font, Casadevall, Martín and Gómez2015), is leading to new sources of oil for local communities.
On the other hand, sea warming threatens cold-water species in the colder places of the Mediterranean such as the Gulf of Lions. This leads to declining populations (e.g. Molva macrophthalma, Sardina pilchardus and Engraulis encrasicolus), range contractions (i.e. retraction to higher latitudes or deeper waters where waters are colder as with Sprattus sprattus), or even forcing local extinctions of certain boreal fish species such as Molva molva (Lloret et al., Reference Lloret, Sabatés, Muñoz, Demestre, Solé, Font, Casadevall, Martín and Gómez2015). In the North Sea and Baltic Sea, this is leading to the replacement of cold-water assemblages, typically characterized by Atlantic herring and European sprat from the 1960s to 1980s, with warmer-water assemblages including Atlantic mackerel, Atlantic horse mackerel, European pilchard and European anchovy from the 1990s onwards (Montero-Serra et al., Reference Montero-Serra, Edwards and Genner2015). Nevertheless, the replacement of typical, cold-water fauna by warm-water species may result in suspicion of a non-traditional fish or low appreciation by consumers of the thermophilic species despite some possibly constituting a good source of fish oil. This is the case for Coryphaena hippurus in the northern Catalan Coast, where this relatively ‘new’ species has a lower market value (it sells for ~€1.5 kg−1 at auction in the fish markets of several ports of this area) compared with the southern Catalan Sea (~€3.5 kg−1) and the Balearic Islands (~€6–9 kg−1), where it has been historically more common and appreciated. This example shows that consumers often do not fully understand or appreciate the gastronomic value of marine exotic species, a fact that also occurs in freshwater ecosystems with the introduction of exotic species for aquaculture purposes (Bartley et al., Reference Bartley, Bhujel, Funge-Smith, Olin and Phillips2005). Thus, we must take careful account of cultural histories and traditions. Such worries can be eased, as for example in the eastern Mediterranean with Lessepian fish that originated from the Red Sea but now are accepted as a normal part of the diet following their migration through the Suez Canal to the Mediterranean (Öztürk, Reference Öztürk2010).
Indirect impacts caused by sea warming to consumers
Predation of immigrated warm-water species may alter the local fauna, not only because they change the abundance of these fauna through competition but also because they may negatively affect fish condition. Where there is a high abundance of predators, prey must spend more time avoiding predators and can forage less often and over larger areas (Walsh et al., Reference Walsh, Hamilton, Ruttenberg, Donovan and Sandin2012). In addition, prey exposed to higher predation risk has higher mass-specific metabolic rates, resulting in more energy being required for maintenance (Walsh et al., Reference Walsh, Hamilton, Ruttenberg, Donovan and Sandin2012). Overall, behavioural or physiological changes in prey under high predator conditions may result in lower net energy intake, which may translate into lower mass gain or storage of energy in fat reserves (Garvey et al., Reference Garvey, Ostrand and Wahl2004). This is an example of the complex links between climate change and fish condition, which needs further investigation in order to assess its ubiquity.
Sea warming is also exerting an indirect effect on fish lipid reserves, as rising seawater temperatures change primary and secondary production, e.g. the abundance and quality of the plankton fish feed upon. For example, climatic conditions partly determine feeding conditions for capelin (Mallotus villosus) and in this way influence both population biomass accumulation and fish fat content (Ellingsen et al., Reference Ellingsen, Dalpadado, Slagstad and Loeng2008; Orlova et al., Reference Orlova, Rudneva, Renaud, Eiane, Vladimir and Alexander2010). Climate shifts could also change essential fatty acid production either by changing phytoplankton species composition or by changing essential fatty acid production within phytoplankton taxa. One of the best-studied examples of changing essential fatty acid production in response to environmental change comes from the Baltic Sea, where eutrophication has shifted phytoplankton dominance from diatoms to flagellates, apparently resulting in changes in essential fatty acid ratios throughout the food web, and possibly leading to a chronic reproductive disease in Atlantic salmon (Salmo salar; Ahlgren et al., Reference Ahlgren, Nieuwerburgh, Wänstrand, Pedersén, Boberg and Snoeijs2005).
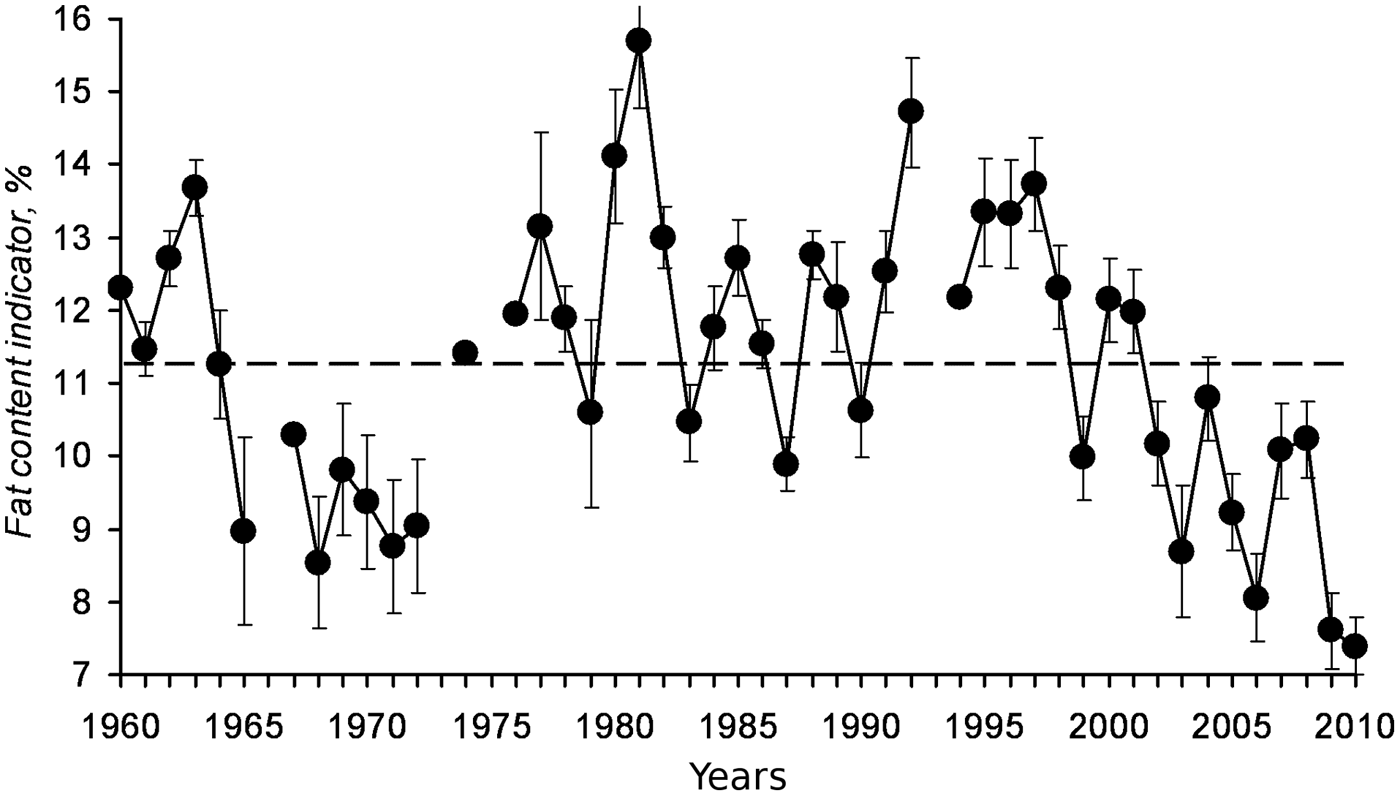
Another example of how sea warming negatively affects the condition of fish through changes in the plankton communities is sprat from the Black Sea, where higher temperatures coincide with a decrease in the biomass of the cold-tolerant complex of phytoplankton, a decrease of mesozooplankton biomass, the main food of sprat, and a simultaneous decline of the abundance, biomass and the individual fat content of this small pelagic fish (reviewed by Lloret et al., Reference Lloret, Shulman and Love2014). The decline in the individual fat content of sprat in the Black Sea from the late 1990s (Figure 2; Nikolsky et al., Reference Nikolsky, Shchepkina, Yuneva and Shulman2011) provides a clear example of how sea warming can reduce the omega-3 supply available to consumers from local cold-water pelagic fish. Also, Litzow et al. (Reference Litzow, Bailey, Prahl and Heintz2006) indicated that changes in lipid content of different fish communities in the Pacific were the result of climate-mediated changes in the availability of essential fatty acids. Following the 1970s Pacific Decadal Oscillation regime shift, walleye pollock Theragra chalcogramma and other demersal lipid-poor species increased in abundance in four boreal zones (Bering Sea, Gulf of Alaska, North Sea and Scotian shelf), while small pelagic lipid-rich species such as capelin, declined (Litzow et al., Reference Litzow, Bailey, Prahl and Heintz2006). A similar situation is recognized in the Gulf of Alaska, where a climate regime shift in the 1970s resulted in population decreases in lipid-rich pelagic species (capelin Mallotus villosus and Pacific herring Clupea pallasii) together with steady population increases in lipid-poor gadoids and pleuronectids (Anderson & Piatt, Reference Anderson and Piatt1999; Mueter & Norcross, Reference Mueter and Norcross2000). In other areas, sea warming did not affect directly the plankton communities that serve as food to fish, but indirectly through the effect of jellyfish. In the Black Sea, the outbreaks of medusa Aurelia aurita in the beginning of the 1980s and the mass invasion at the end of the 1980s by the ctenophore Mnemiopsis leidyi, reduced the prey available for planktivorous fishes and decreased sprat fatness at the end of the feeding period from 15.5 to 9% (reviewed by Lloret et al., Reference Lloret, Shulman and Love2014). Mnemiopsis had an even higher impact on anchovy in the Sea of Azov, where fat content decreased from 20–30% to 10–15%, resulting in the collapse of the fishery (reviewed by Lloret et al., Reference Lloret, Shulman and Love2014).

Fig. 2. Fat content indicator (% lipid wet weight) in Black Sea sprat from 1960 to 2011 (from Nikolsky et al., Reference Nikolsky, Shchepkina, Yuneva and Shulman2011).
Implications of sea warming for seafood safety
Pathogens, contaminants and biotoxins are key to the safety of seafood as they pose health risks to consumers that are expected to increase due to sea warming, leading to illness in humans and other organisms (European Marine Board, 2013). Biotoxins provide a good example of the increasing threats arising from sea warming. Under favourable warmer conditions, some species of phytoplankton that produce potent biotoxins grow rapidly and multiply causing ‘blooms’ (so-called harmful algal blooms or HAB; see Berdalet et al., Reference Berdalet, Fleming, Gowen, Davidson, Hess, Backer, Moore, Hoagland and Enevoldsen2015), which can cause adverse health effects to wildlife through oxygen depletion leading to mass mortality of marine living resources, and to humans through the consumption of contaminated seafood leading to damage to the liver and nervous system (Moore et al., Reference Moore, Trainer, Mantau, Parker, Laws, Backer and Fleming2008; Berdalet et al. Reference Berdalet, Fleming, Gowen, Davidson, Hess, Backer, Moore, Hoagland and Enevoldsen2016). Harmful algal blooms are projected to increase in frequency and intensity, in part due to climate warming, together with increased microbial pollution from coastal populations and the resulting nutrient load (European Marine Board, 2013). Whereas warmer temperatures create a competitive advantage for certain types of harmful algae which favours their growth, other factors linked to climate change such as increases in dissolved carbon dioxide in marine ecosystems, coastal waters associated with sea level rises, and increased river runoff (and associated anthropogenic nutrients) may favour the growth of harmful algae (EPA, 2013). It is also relevant to note that this is often site-specific, with local hydrodynamic processes often determining whether or not blooms occur (Davidson et al., Reference Davidson, Gowen, Harrison, Fleming, Hoagland and Moschonas2014).
Another example of expanding biotoxin presence linked to sea warming is the case of lipid-soluble ciguatoxins produced by dinoflagellates (microalgae) of the genus Gambierdiscus that accumulate in some thermophilic fish, producing the so-called ‘ciguatera fish poisoning’, a seafood-borne illness that has become a hazard to consumers in non-endemic regions such as the USA and Germany (Dickey & Plakas, Reference Dickey and Plakas2010). The expansion of ciguatera is due not only to the expanding international trade in seafood from tropical fisheries (Dickey & Plakas, Reference Dickey and Plakas2010) but also because sea warming has contributed to the emergence of toxic dinoflagellate species and ciguatoxic fish in subtropical and even temperate regions that previously had been restricted to tropical areas (Mattei et al., Reference Mattei, Vetter, Eisenblatter, Krock, Ebbecke, Desel and Zimmermann2014). Here, immigrating ciguatoxic, thermophile fish species, moving from their original subtropical and tropical habitats to temperate areas add risks to human health and well-being in these new localities. A growing number of ciguatera poisoning cases are being reported in Europe and the presence of Gambierdiscus spp. both in the Mediterranean Sea and Canary Islands has been linked to the spread of toxic dinoflagellates and ciguatoxic fish, suggesting that this problem is already affecting regions in more temperate latitudes (Otero et al., Reference Otero, Pérez, Alfonso, Vale, Rodríguez, Gouveia, Gouveia, Delgado, Vale, Hirama, Ishihara, Molgó and Botana2010; Alverca, Reference Alverca2011). In the Canary Islands, for example, at least three ciguatera outbreaks have been reported since 2010, which coincided with the detection of novel toxin-producing dinoflagellates (Boada et al., Reference Boada, Zumbado, Luzardo, Almeida-Gonzalez, Plakas, Granade, Abraham, Jester and Dickey2010; Nuñez et al., Reference Nuñez, Matute, García, García and Abadía2012). In the Mediterranean, reports suggest the presence of ciguatoxin-like substances in the Lessepsian rabbitfish Siganus rivulatus in the eastern Mediterranean (Bentur & Spanier, Reference Bentur and Spanier2007). Additionally, during the last 15 years, reports of toxic episodes involving benthic dinoflagellates belonging to the ciguatera community, in particular Ostreopsis, have shown a marked increase in warm-temperate areas particularly the Mediterranean Sea, affecting several countries including Spain, Italy, France, Greece and more recently Portugal (Alverca, Reference Alverca2011).
Another example of an expanding toxin linked to sea warming is tetrodotoxin (TTX), a potent neurotoxin produced by bacteria that is found in liver, gonads and gastrointestinal tract and skin of some puffer fish (Lagocephalus spp.) and some other marine organisms (Lee et al., Reference Lee, Jeong, Kim, Kim, Kim, Park, Park, Kim, Kim and Kim2000). Puffer fish are marine species that are distributed in tropical and subtropical areas of the Atlantic, Indian and Pacific Oceans that are spreading to temperate areas as sea temperatures rise, with the consequent spread of the tetrodotoxin that some species contain (see e.g. Golani, Reference Golani, Golani and Appelbaum-Golani2010). For example, a puffer fish (Lagocephalus sceleratus) invasion in the eastern Mediterranean is becoming a serious hazard to consumers due to tetrodotoxin (Sabrah et al., Reference Sabrah, El-Ganainy and Zaky2006; Katikou et al. Reference Katikou, Georgantelis, Sinouris, Petsi and Fotaras2009), with poisoning incidents following consumption of L. sceleratus being reported in some countries (see e.g. Bentur et al., Reference Bentur, Ashkar, Lurie, Levy, Azzam, Litmanovich, Golik, Gurevych, Golani and Eisenman2008; Katikou et al., Reference Katikou, Georgantelis, Sinouris, Petsi and Fotaras2009).
Despite the alarming health hazards associated with the spread of ciguatera and tetrodotoxin-toxic thermophilic fish, it seems that the negative ecological and fishery effects of these fish once they enter the new habitat exceeds the health hazards. For example, an important economic impact of puffer fish in the eastern Mediterranean is to the small-scale local fishery sector, as the puffer fish feeds on commercial species entangled in nets, leading to significant losses of income and damage to fishing gear (Rousou et al., Reference Rousou, Gania, Kletou, Loucaides and Tsinganis2014). Similarly, rabbitfish in the eastern Mediterranean has profound negative effects on algal forests, which are among the most productive and diverse communities of temperate Mediterranean coasts, providing resources including food and habitat for large numbers of exploited fish and invertebrates (Vergés et al., Reference Vergés, Tomas, Cebrian, Ballesteros, Kizilkaya, Dendrinos, Karamanlidis, Spiegel and Sala2014). These ecological/fishery effects have the potential to raise significant seafood security concerns in developing nations of the eastern Mediterranean.
Rising temperatures are also associated with changes in the distribution and occurrence of pathogens found in fish such as microbes and parasites that are harmful for consumers. During the last half century, ocean warming has favoured the spread of marine bacteria of the Vibrio genus, including Vibrio cholera (which can cause cholera in humans) and may be the cause of the globally increasing trend in their associated diseases (Vezzulli et al., Reference Vezzulli, Brettar, Pezzati, Reid, Colwell, Höfle and Pruzzo2012). There is also a clear effect of increased sea temperatures on the growth rates of parasites in fish hosts (Macnab & Barber, Reference Macnab and Barber2012). The potential ‘booster’ effect of sea warming on parasites can lead to additional worries for the consumer.
Finally, the bioavailability and toxicity of some contaminants found in fish such as mercury and persistent organic pollutants (POPs) is likely to increase in response to rising water temperatures, and hence the health risk for consumers (see e.g. Noyes et al., Reference Noyes, McElwee, Miller, Clark, Van Tiem, Walcott, Erwin and Levin2009; Dijkstra et al., Reference Dijkstra, Buckman, Ward, Evans, Dionne and Chen2013). Recent studies show that warmer sea temperatures may increase the ability of marine fish to accumulate mercury because fish in warmer water eat more and have higher methylmercury levels in their tissues, suggesting that increases in their metabolic rate causes the increased mercury uptake (Dijkstra et al., Reference Dijkstra, Buckman, Ward, Evans, Dionne and Chen2013). Although still little is known about the interactions between temperature and other contaminants such as persistent organic pollutants that are found in exploited marine fish (Ross & Birnbaum, Reference Ross and Birnbaum2003), studies conducted in freshwater ecosystems demonstrated that the lethality of the persistent organic pollutant dieldrin and the toxicity of herbicide atrazine to freshwater fish increased with increasing temperatures (reviewed by Noyes et al., Reference Noyes, McElwee, Miller, Clark, Van Tiem, Walcott, Erwin and Levin2009), raising concerns that a similar effect may also occur in marine fisheries.
Impact of river runoff on fish condition and health
Apart from sea warming, other factors linked to climate change such as changes in river runoff, salinity and winds, coastal flooding associated with sea-level rise, increased storminess and decreasing dissolved oxygen all affect marine biota (Whitney et al., Reference Whitney, Freeland and Robert2007; Stramma et al., Reference Stramma, Johnson, Sprintall and Mohrholz2008; Denman et al., Reference Denman, Christian, Steiner, Portner and Nojiri2011; Icarus-Allen, Reference Icarus-Allen, Hester and Harrison2011). Among these factors, river runoff is probably an overlooked factor that is posed to impinge on exploited fish condition and health in coastal areas. In many tropical and subtropical regions such as the Mediterranean Sea, southern North America and Central America, river runoff is expected to decrease following a decline in rainfall and/or snowfall (Nohara et al., Reference Nohara, Kitoh, Hosaka and Oki2006), combined with the increasing human economic demands for agriculture, livestock production, various industries and hydroelectric power generation (Gleick, Reference Gleick2004). Lower riverine water inputs will affect oil reserves of pelagic fish inhabiting these southern seas through a reduction in plankton productivity (a consequence of lower river nutrient input). In contrast, in high-latitude rivers, riverine run-off is expected to increase as a consequence of climate change (Nohara et al., Reference Nohara, Kitoh, Hosaka and Oki2006), which could enhance pathogen pollution in the marine environment. For example, river run-off is a source of the protozoan Toxoplasma gondii, a recognized pathogen of humans as well as terrestrial and marine animals including fish (Massie et al. Reference Massie, Ware, Villegas and Black2010). Increased river run-off in these northern areas will also add nutrients to coastal waters, which, together with higher light intensities and temperatures, may increase the growth of toxic algal and cyanobacterial blooms (Shaw et al. Reference Shaw, Garnett, Moore and Florian2001), which can lead to fish and shellfish safety concerns as these organisms concentrate the toxic compounds from the blooms, becoming a health hazard for consumers (European Marine Board, 2013).
Ocean acidification
Increased CO2 from fossil fuel emissions enters the ocean and makes it more acidic (the pH decreases). When pH decreases, the carbon state, i.e. the balance between bicarbonate and carbonate in the ocean, changes so that there is less carbonate. This shift has important implications for plants and animals that build calcium carbonate (CaCO3) structures (Haigh et al., Reference Haigh, Ianson, Holt, Neate and Edwards2015). Calcifying organisms such as molluscs and crustaceans comprise a large component of fisheries in many areas including the USA and Canada, where these animals can represent more than 65% of the total landed value (Cooley & Doney, Reference Cooley and Doney2009; Haigh et al., Reference Haigh, Ianson, Holt, Neate and Edwards2015). Furthermore, ocean acidification may affect the phytoplankton and zooplankton communities – particularly copepods and pteropods – that form a substantial biomass in the oceans and provide an important source of food for upper trophic levels in temperate marine food webs – and hence indirectly have a negative impact on fisheries (reviewed by Haigh et al., Reference Haigh, Ianson, Holt, Neate and Edwards2015).
Furthermore, with ocean acidification, some toxic phytoplankton species may gain a competitive advantage and threaten wild and cultured fish, e.g. Heterosigma akashiwo in the North-east Pacific, a raphidophyte that grows faster when dissolved CO2 is higher, releasing peroxide free radicals that damage fish gills and cause significant mortality and monetary losses (Haigh et al., Reference Haigh, Ianson, Holt, Neate and Edwards2015). In a similar way, increasing partial pressure of carbon dioxide (PCO2) has been shown to alter the mix of neurotoxins produced by toxic alga genera such as Pseudo-nitzschia and Alexandrium to favour more potent forms, posing a significant threat to higher trophic levels impacting the shellfish industry as well as overall food safety (Haigh et al., Reference Haigh, Ianson, Holt, Neate and Edwards2015). Furthermore, ocean acidification may also change the structure and composition of macroalgae and macroinvertebrate communities that provide food and essential habitats for commercially important fish species such as the calcified red algal deposits known as maërl beds (Biomaerl Team, 2003), the free-swimming echinoderms known as crinoids (Colloca et al., Reference Colloca, Carpentieri, Balestri and Ardizzone2004), coralligenous assemblages (Ballesteros, Reference Ballesteros2006) and coral reefs (Kleypas & Yates, Reference Kleypas and Yates2009).
However, there are still significant knowledge gaps on the nature and degree of these effects, particularly regarding the indirect effects of ocean acidification on particular life stages of exploited species and the prey they feed on, in particular the combined effects of multiple stressors such as climate change, chemical contamination and HABs (Denman et al., Reference Denman, Christian, Steiner, Portner and Nojiri2011; Haigh et al., Reference Haigh, Ianson, Holt, Neate and Edwards2015). In this sense, it is impossible to predict potential changes to exploited organisms due to a single factor alone (e.g. decreasing pH/increasing PCO2) when they are subjected to simultaneous changes in other variables that are expected to change with the climate such as increasing temperature and decreasing dissolved oxygen (Denman et al., Reference Denman, Christian, Steiner, Portner and Nojiri2011).
CONCLUSIONS AND RECOMMENDATIONS
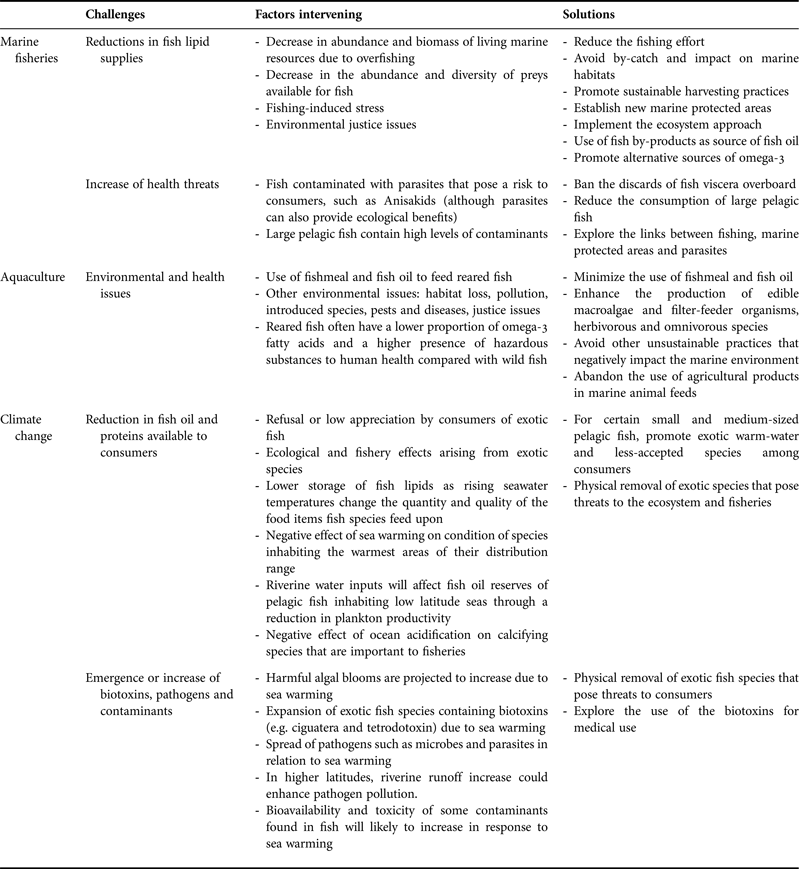
Fishing activities, aquaculture and climate change are challenging the benefits and risks of marine resources to human health and well-being (Table 1; Figure 3). These challenges will have increasing global implications taking into account the advancing worldwide demand for seafood products, and are therefore becoming a central issue in in the concept of ‘Oceans & Human Health’. Seafood provides a good example of how oceans and human health are inter-connected, highlighting both the benefits and risks from fishing, aquaculture and climate change. In this context, we put forward several recommendations that could help minimize the effects of global change on fish security and safety, and therefore on human health and well-being.

Fig. 3. Links between seafood, fishing, aquaculture, climate change and human health.
Table 1. Summary of the main challenges regarding the links between seafood and human health and well-being, the factors intervening and the possible solutions.

Move towards better management of fisheries
Adoption of better management procedures will contribute to sustainable fishing practices that avoid overexploitation and support stock recovery. Although climate change (sea warming, ocean acidification, etc.) cannot be changed by fisheries management, the negative effects can be reduced if resilient (healthy) fish stocks are maintained through better management practices. These practices include: (a) the adaptation of fishing capacity to sustainable resource productivity; (b) the promotion of environmentally friendly fishing techniques that minimize discards and avoid degradation and loss of marine habitats and biodiversity; (c) the promotion of integrated ecosystem approaches (ecosystem-based fisheries management); (d) the establishment of new marine protected areas to increase not only fish abundance, biomass and biodiversity, but also to enhance fish lipid reserves (with positive consequences for the ecosystems and human health); and (e) the promotion of sustainable harvesting practices that allow individual replacement associated with the maximum catch in the longer-term perspective. Such advice would follow the three rules summarized by Froese (Reference Froese2004): let fish spawn (the target would be to let all fish spawn at least once before they are caught to rebuild and maintain healthy spawning stocks), let fish grow (the target would be to catch all fish at sizes close to their optimum weight increment, i.e. a size selectivity where the maximum yield or revenue can be obtained, and at the same time allow the megaspawners to live (Birkeland & Dayton, Reference Birkeland and Dayton2005; Caddy, Reference Caddy2015). For sex-changing species (i.e. species that change their sex at a certain size), it is also important to let both sexes live and thus not exploit one sex alone with the target to avoid size-selective fisheries that disproportionately remove individuals of one sex, leading to biased sex ratios (Alonzo & Mangel, Reference Alonzo and Mangel2004).
Promotion of sustainable aquaculture practices
In order to attain sustainable aquiculture, it is important to avoid practices that negatively impact the marine environment (habitat loss, pollution, introduced species, pests and diseases), and to minimize the unsustainable use of fishmeal and fish oil. Currently fish oil is the only economically viable source of long-chain omega-3 fatty acids for feed purposes in aquaculture (FAO, 2014). There are no good alternative sources of eicosapentaenoic and docosahexaenoic acids for feeding cultured fish at present because alternatives such as microalgae are too costly for feed purposes and not a viable option in the near future (FAO, 2014). Therefore, three possible approaches shall be considered in this context. First, the promotion of direct human consumption of the fish used to produce fishmeal (rather than the consumption of reared fish); this would be not only healthier but also ecologically sound. Second, mariculture must abandon its current dependence on fisheries catches for producing fish meal and fish oil and instead encourage the production of edible macroalgae, filter-feeder organisms and herbivorous or omnivorous fish, which use much less fishmeal than do carnivorous species per tonne of protein. Third, mariculture should also abandon the use of agricultural products in marine animal feeds, which represents an indirect use of cropland and water and leads to competition between fish and humans for food (Duarte et al., Reference Duarte, Holmer, Olsen, Soto, Marbà, Guiu, Black and Karakassis2009).
Landing and use of fish-by-products
Livers and intestines, where a number of fish species store lipids (liver oil and perivisceral fat, respectively), are usually discarded as waste. Only the livers from some species (e.g. cod) are used to produce fish oil (omega-3) capsules for human consumption. If liver and perivisceral fats of more species could be used more effectively, sources of omega-3 would be greatly enhanced. However, since many fish accumulate pollutants and parasites in their viscera, or even biotoxins such as TTX and ciguatera, the use of viscera should be carried out under strictly controlled product regulations. This procedure would also contribute to the management practices required in commercial fishing needed to ensure healthy fish stocks (McClelland, Reference McClelland2002; Lloret, Reference Lloret2010). For example, viscera of some Mediterranean groundfish species, such as the catshark Scyliorhinus canicula and Lophius spp., are often discarded at sea prior to reaching the fish market. This practice may result in heavier parasite loads in fish that feed on the discarded viscera (McClelland, Reference McClelland2002). Therefore, a solution would be to ban the discards of fish viscera overboard and their re-use promoted (under strict control rules to ensure their safety).
Promotion of warm-water species among consumers
For certain small and medium-sized pelagic fish, the promotion of warm-water (often under-exploited) and less accepted species among consumers could ease the pressure on cold-water (often over-exploited), traditional species. For example, in the north-western Mediterranean the consumption of warm-water species, such as Sardinella aurita, Trachinotus ovatus and Sphyraena viridensis, which are increasing due to sea warming (Lloret et al., Reference Lloret, Sabatés, Muñoz, Demestre, Solé, Font, Casadevall, Martín and Gómez2015), could be promoted as a substitute for cold-water species that suffer from overfishing and sea warming, such as Engraulis encrasicolus and Sardina pilchardus (Martín et al., Reference Martín, Sabatés, Lloret and Martín-Vide2010). However, this suggestion must be cautiously considered and on a case-by-case basis. For example, it might not be suitable in the case of certain warm-water species such as thermophilic groupers or billfishes, which are also spreading into new areas because of sea warming but have life history traits that render them very vulnerable to fishing (see e.g. Lloret et al., Reference Lloret, Sabatés, Muñoz, Demestre, Solé, Font, Casadevall, Martín and Gómez2015).
Promotion of alternative/complementary sources of omega-3
The overexploitation of certain fish stocks highlights the urgent need to seek alternative sources of omega-3 fatty acids such as marine algae, microorganisms and plants (Surette, Reference Surette2008). Cultivated algal oils, for example, are free of contaminants and satisfy ethical considerations, but their value as a health asset remains to be demonstrated (Brunner et al., Reference Brunner, Jones, Friel and Bartley2009) through long-term analyses. In addition, strict monitoring of algae producers by health authorities will be needed to assure adequate protection for consumers. Blue-green algae (cyanobacteria) Spirulina spp., often used to produce food supplements proposed as health-promoting natural products, are usually collected from the natural environment where other potentially toxic cyanobacteria such as Microcystis aeruginosa can be present (Draisci et al., Reference Draisci, Ferretti, Palleschi and Marchiafava2001). This raises the issue of potential contamination of dietary supplements by cyanotoxins such as anatoxins and microcystins, with associated risks for human health since these compounds are neurotoxic (Drobac et al., Reference Drobac, Tokodi, Simeunović, Baltić, Stanić and Svirčev2013).
Reduction in consumption of large pelagic fish
It is important to reduce the consumption of large pelagic fish such as swordfish and tuna species which, besides being overexploited due to high demand, may contain high levels of contaminants, particularly mercury (European Marine Board, 2013). In fact, species deemed unsustainably fished have significantly higher levels of mercury but do not provide higher levels of long-chain omega-3 fatty acids (Gerber et al., Reference Gerber, Karimi and Fitzgerald2012). Thus, the reduction in consumption of these species would have benefits for both human health and marine ecosystems. It is perhaps impossible to consume fish and have no risk of methylmercury exposure, but if high methylmercury containing species are substituted with low methylmercury containing species, methylmercury exposure could be minimized while retaining the beneficial health aspects associated with fish consumption (Johnston & Snow, Reference Johnston and Snow2007), and at the same time avoid pressures on unsustainable seafood (Gerber et al., Reference Gerber, Karimi and Fitzgerald2012).
Mitigation actions to control the expansion of toxic exotic fish
Some exotic fish are toxic and threaten the health of consumers and sometimes indigenous fish communities too if they are invasive. Then, strategies must be considered to limit their impact, for example the physical removal (catch) of invasive species that pose threats to consumers, such as puffer fish. Authorities in Cyprus offered compensation of €1–3 per puffer fish to local fishermen in 2010 and this resulted in the catch of massive amounts of puffer fish that were later destroyed at a local authorized incineration unit (Rousou et al., Reference Rousou, Gania, Kletou, Loucaides and Tsinganis2014). However, it should be noted that control of non-indigenous populations that have a spatially broad ecological biotope can be expensive as well as ineffective. Another possibility would be exploration of the use of biotoxins such as tetrodotoxin for medical use (Rousou et al., Reference Rousou, Gania, Kletou, Loucaides and Tsinganis2014), which could foster the catch/removal of these toxic species.
The need for multidisciplinary research on oceans and human health
More multidisciplinary research is needed to enhance our understanding of the ecological mechanisms behind seafood security and safety concerns. Additional multidisciplinary investigations should cover the impact of sea warming and fishing on fish oil reserves as well as the origin and dynamics of toxins in seafood (e.g. tetrodotoxin and ciguatera), health benefits of fish consumption, social and environmental equity issues arising from projected demands for caught and farmed fish, direct and indirect impacts of ocean acidification on fishery species, combined effects of multiple stressors (e.g. temperature–contaminant interactions), as well as effects of fishing on parasite abundance and diversity and the role of parasites in the efficacy of marine reserves.
ACKNOWLEDGEMENTS
The authors would like to thank Lora E. Fleming, Michael Thorndyke, Fiona McGowan, Ann Pulsford and Helena Solo-Gabriel for providing valuable comments and corrections that have helped to improve the text.