Aboriginal and Torres Strait Islander peoples continue to experience high rates of CVD, diabetes and cancer and higher mortality rates compared with non-Indigenous Australians(1), noting the social and cultural determinants of health, along with the complex and historical factors of settlement and colonisation that underlie these conditions(Reference Rowley, O’Dea and Anderson2).
The starkest population health differential is observed for chronic diseases such as type 2 diabetes, CVD and renal disease, where the prevalence of diabetes and heart disease is up to ten times higher among Aboriginal peoples compared with the non-Indigenous population(1,Reference Wilson, Zhao and Condon3) .
Well-established traditional risk markers for diabetes and CVD including high glycated Hb (HbA1c), hyperglycaemia and high blood pressure have been reported as more prevalent among Aboriginal and Torres Strait Islander peoples compared with non-Indigenous Australians(Reference Brown, Carrington and McGrady4).
Additional risk factors including obesity (high BMI, waist-to-hip ratio and waist-to-height ratio), high albumin/creatinine ratio (ACR), high-sensitivity C-reactive protein (hs-CRP) and cholesterol levels have also been reported to play a significant role as CVD and diabetes risk markers in this specific population(Reference Brown, Carrington and McGrady4–Reference Strate, Brimblecombe and Maple-Brown6).
Added to this burden of non-communicable risk and disease are high rates of communicable diseases, such as rheumatic heart disease, skin, ear, nose and throat infections, caused by overcrowded housing among other structural factors. Recurrent infections can synergistically exacerbate chronic disease processes and result in chronic high inflammatory load, which can be measured by plasma pro-inflammatory marker hs-CRP(Reference Shemesh, Rowley and Jenkins7,Reference Arnold, Hoy and Wang8) .
Nutrition, which is one of the important modifiable determinants of the development and progression of these diseases, has been estimated to account for almost 10 % of the total burden of disease in this population(9).
Recent reports on health status among Aboriginal peoples in Australia describe many as not meeting dietary recommendations according to national guidelines(Reference Lee and Ride10–12), similar to the rest of the Australian population. The recommended intake of vegetable was met by 4·4 % Aboriginals and Torres Strait Islander adults compared with 6·8 % non-Indigenous adults while recommended fruit intake was met by 54 % for both(13).
Aboriginal and Torres Strait Islanders’ self-provisioning food production and procurement methods are characterised by high physical activity and consumption of a wide range of nutrient-dense foods, including wild plant varieties containing higher levels of vitamins and dietary fibre than modern cultivated plant foods(Reference O’Dea14). However, this traditional food system is today integrated with a more energy-dense ‘westernised’ diet, high in fat and refined sugars, with inadequacies in nutritional intake influenced by availability and affordability caused through socio-economic, environmental and geographic factors(Reference O’Dea14–16). The majority of communities in remote areas of Australia access food from community stores. These remote community stores operate across great tracts of very sparsely populated areas in Australia and provide a range of food options, albeit at high costs compared with urban supermarkets. High food costs coupled with low incomes and limited choice have been found to promote consumption of less nutrient-dense foods, such as high fat takeaway foods and sugar-sweetened beverages(Reference O’Dea14–16). These changes in dietary quality with settler colonisation and socio-economic disadvantage have impacted on Indigenous populations worldwide(Reference Kuhnlein, Receveur and Soueida17).
Plasma antioxidants are recognised as useful biomarkers of fruit and vegetable intake(Reference Coyne, Ibiebele and McNaughton18–Reference Souverein, de Vries and Freese20) and diet quality(Reference Nybacka, Lindroos and Wirfält21,Reference Fallaize, Livingstone and Celis-Morales22) . Previous studies in Aboriginal and Torres Strait Islander communities have examined plasma antioxidants and the association with microalbuminuria(Reference Rowley, O’Dea and Su23), hs-CRP and vascular endothelial activation with the presence and absence of metabolic syndrome(Reference Rowley, Walker and Cohen24) and absence of diabetes, chronic kidney disease, CVD and hypertension(Reference Luke, Ritte and O’Dea25). Many of these studies reported low carotenoid levels in Aboriginal communities in rural and remote northern and central Australia(Reference Rowley, O’Dea and Su23,Reference Luke, Ritte and O’Dea25,Reference Rowley, Su and Cincotta26) , and importantly Luke et al.(Reference Luke, Ritte and O’Dea25) reported a protective effect against CVD at 10 years follow-up among those with higher carotenoid levels at baseline. No previous studies have examined the association between cardiometabolic risk profile based on several cardiometabolic risk markers and plasma antioxidants in an Aboriginal population in Australia. Thus, we aimed to examine the cross-sectional association between plasma antioxidants and cardiometabolic risk in an Aboriginal population in remote Northern Territory, Australia, to gain more knowledge on the influence of antioxidants and risk markers before the establishment of cardiometabolic diseases and to strengthen the evidence on how to prevent the high burden of chronic diseases in this population.
Methods
This was a sub-study within a community-initiated health promotion study that occurred in a remote community in Northeast Arnhem Land in Northern Territory, Australia during the periods August–September 2001 and February–March 2002(Reference Strate, Brimblecombe and Maple-Brown6,Reference Brimblecombe, Mackerras and Garnggulkpuy27) . The study drew on community-based participatory research approaches and was conducted in a two-way learning partnership with the community and a local organisation participating in all aspects of the study processes. This included in-kind and paid support with recruitment, data collection and feedback to study participants and the wider community to ensure relevance to the community and respectful study conduct according to the national guidelines for ethical conduct in Aboriginal and Torres Strait Islander health research(28), and thus ensured high quality data.
This sub-study used the data collected to examine the association between plasma antioxidants and cardiometabolic risk to help inform community and government policy approaches to health improvement. This article was presented to the community research organisation and other community organisations in December 2019.
The study population, recruitment and health screening have been described in detail elsewhere(Reference Brimblecombe, Mackerras and Garnggulkpuy27). Briefly, all residents aged ≥ 15 years were invited to participate through local posts and newspapers and elders and community leaders communicated their support of the project across the community and homelands. Interested and eligible residents were given written and verbal information about the study before giving informed consent to participate. Additional informed consent was obtained from parents of participants below 18 years.
The present study is based on all participants with complete measurements of anthropometric measurements, fasting blood samples (including lipid-soluble antioxidants) and urine samples, which were collected and completed in 333 among the identified 706 residents of 15 years or older in a house-to-house census in 2002. Of these, nine reported to be pregnant and were subsequently excluded from the present study. Therefore, the final sample for this particular study was 324 participants (147 men and 177 women).
Measurements
Cardiometabolic risk markers were examined based on anthropometric and blood pressure measurements, overnight fasting blood and urine samples.
The anthropometric measurements included measurements of weight, height, waist and hip circumference. Body weight was measured to 0·1 kg using a digital portable scale and height to the nearest 0·1 cm using a wall-mounted stadiometer, and BMI was calculated as (kg/m2). Waist and hip circumference were measured using standard anthropometric techniques, waist-to-hip-ratio as waist circumference in cm/hip circumference in cm and waist-to-height-ratio was calculated as waist in cm/height in cm.
Blood pressure was measured after 5 min rest (seated) using an automated Dynamap monitor (Critikon XL; Johnson & Johnson). Blood pressure was measured three times. The first measurement was disregarded, while the second and the third were averaged.
Blood samples were obtained for measurements of glucose, lipids and plasma antioxidants. The blood samples were collected by venepuncture early in the morning after an overnight fast and kept on ice until centrifugation (< 6 h later). Up to 13 ml of blood was collected in tubes appropriate for each assay. Blood samples were stored on ice until centrifuged at 3000 RPM for 15 min. Plasma was then separated and frozen immediately at –20 °C until transfer to a storage at –80 °C (< 2 weeks) and analysed at Flinders Medical Centre, Adelaide accredited in the US Centre for Disease Control Lipid for Standardisation Program.
Plasma glucose was measured using standard enzymatic analyses by a Hitachi 917 analyser (Abbott Axsym). Aliquots of whole blood for glycated Hb (HbA1c) were taken from EDTA tubes and HbA1c was measured on a Pharmacia Mono S instrument.
Hs-CRP was assayed using a high sensitive commercial assay (BM-II nephelometer; Dade Behring Diagnostics: intra- and inter-assay CV 1·9 and 4·4 %).
Diet quality was measured by biomarkers of diet quality, lipid-soluble plasma antioxidants including plasma carotenoids (β-cryptoxanthin, β-carotene, lycopene, lutein-zeaxanthin) and antioxidant vitamins (α-tocopherol and retinol), which were extracted and assayed by HPLC as described previously(Reference Su, Rowley and O’Dea29). Briefly, 200 μl plasma was extracted twice with 1 ml of Hexane containing 0·01 % butylated hydroxytoluene (BHT). For quantification, an internal standard of Echinenone 0·167 μg/ml was added to all samples prior to the extraction. The extract was dried under nitrogen at room temperature, then reconstituted in 100 μl of mixture CHCl3:MeOH:CH3CN(30,Reference Gross, Yu and Hannan35,Reference Gross, Yu and Hannan35) . Fifty μl was injected into the HPLC (Shimadzu HPLC machine equipped with an SPD-M20A PDA Detector and a Novo-Pak C18 column) with absorbance detection at 292 nm for the tocopherols, 325 nm for retinol and 450 nm for the carotenoids. The carotenoids, retinol and tocopherols were eluted from the column using a mobile phase of 0·0125 % ammonium acetate in MeOH (A), 100 % CHCl3 (B) and CH3CN with 0·1 % triethylamine (C) in three linear gradient steps: from 0 to 5 min, A 50 %, C decreased from 50 to 44 % and B increased to 6 %; from 5 to 16 min, A increased to 55 %, C decreased from 44 to 30 % and B increased from 6 to 15 %, washed with A:C 50/50 mixture for 3 min.
Urine samples were assayed for albumin on a Beckman array instrument and for creatinine on a Hitachi 917, ACR was calculated.
Health behaviour questionnaire
A health behaviour questionnaire was co-developed with members of the community research organisation and administrated in local language by community researchers. The questionnaire examined dietary habits by four self-reported food frequency items regarding intake of fruit, vegetables, traditional foods (produced and procured native flora and fauna, hereafter referred to as traditional foods) and takeaway (pre-prepared purchased meals). The food frequency items are accordingly categorised as: ≤ 2–3 times a week; most days or every day, to dichotomise into categories closest to national guidelines recommending daily consumption of fruit and vegetable intake(30).
The health behaviour questionnaire also collected other self-reported personal and lifestyle information: ever had diabetes, gestational diabetes, kidney disease or heart disease (yes, no, do not know); self-reported use of medication as open answers (recoded to medication for diabetes, kidney disease, heart disease or blood pressure yes/no), place of residence (i.e., referred to as living on homeland, which in the current study refers to places where small populations of Aboriginal people live on lands to which they have traditional ownership and/or ancestral associations or in the main town community); employment (working or studying, not working); smoking habits (currently, previously, never); kava (traditionally used depressant derived from the kava plant root and shared, originally from Pacific Islander culture) or alcohol consumption (ever, never); weekly frequency of engaging in physical activities such as sports, hunting, walking for exercise (never or ≤ 4 times a week, more than 4 times a week); whether participants were currently living on their homeland (yes, no).
Statistical analysis
All continuous data are presented as mean (sd) except those with skewed distribution which were log-transformed to approximate normal distributions. Clinical characteristics were calculated for the total study population and by sex for the continuous variables. For log-transformed variables, geometric means and 25th to 75th percentiles were calculated. Differences between men and women were analysed by mean difference and student’s t test. Categorical variables obtained from the health behaviour questionnaire were presented for the total population and χ 2 test used to discern differences between men and women.
Cardiometabolic risk profile was determined by categorising cardiometabolic risk markers into clinically relevant population-specific cut-offs according to the CARPA (Central Australian Rural Practitioner’s Association) Standard Treatment manual, a relevant general practice primary health care manual written for use in remote central and northern Aboriginal communities(31), Maple-Brown et al., 2013(Reference Maple-Brown, Brimblecombe and Connelly32) and Daniel et al., 2002(Reference Daniel, Rowley and McDermott33).
We aimed to investigate antioxidants as a marker of diet quality in general and thus tested if the plasma carotenoids and vitamins were appropriate for principal component analysis (PCA) to reduce the variables into a smaller number of components (details presented in online supplementary materials), previously done in an Indigenous population in Australia(Reference Luke, Ritte and O’Dea25). A correlation matrix with plasma carotenoids and vitamins revealed high inter-correlations (r > 0·3) between the plasma carotenoid and vitamins (β-carotene, β-cryptoxanthin, lycopene, lutein-zeaxanthin, α-tocopherol and retinol). PCA was used to group highly correlated plasma carotenoid and vitamin variables into a smaller number of components(Reference Jolliffe and Cadima34), with an oblimin rotation, one of two oblique rotation methods which assume the factors are correlated, as revealed by the correlation matrix. Kaiser–Meyer–Olkin value and Bartlett’s test of sphericity were assessed, and Kaiser’s criterion and scree plot were used to select the number of components to be extracted. Monte Carlo PCA was used for parallel analysis which was systematically compared with the eigenvalues obtained from the PCA analysis. The component matrix and pattern matrix were also used to decide the number of components to be extracted.
The PCA revealed six component factors: two components with an eigenvalue > 1·0, Kaiser–Meyer–Olkin value = 0·67, exceeding the recommended value of 0·60 and Bartlett’s test of sphericity was statistical significance (P < 0·01), confirming PCA as an appropriate data reduction method. We extracted one component based on the scree plot indicating a break between the first and second component, the component matrix showing unrotated loadings loaded above 0·6 for five items and above four for the last item. The pattern matrix revealed most loadings above 0·4 on component 1, explaining 44 % of the total variance. This component is further referred to as ‘antioxidant component’ variable, which was used for further analysis. Individuals with higher antioxidant component scores indicate higher carotenoids and vitamin levels.
Using t tests, we examined whether the component variable differed across categories of self-reported dietary intake and smoking. The antioxidant component was further examined as an explanatory variable in stepwise linear regressions to assess the association with cardiometabolic risk variables (BMI, waist circumference, waist-to-hip ratio, waist-to-height ratio, systolic and diastolic blood pressure, fasting plasma glucose, HbA1c, hs-CRP, ACR).
The stepwise linear regressions included unadjusted models, models adjusted for sex, age, smoking status and total cholesterol as in previous studies(Reference Rowley, Walker and Cohen24,Reference Gross, Yu and Hannan35) , as these are associated with the cardiometabolic risk markers (the exposure)(Reference Gepner, Piper and Johnson36,Reference Klein, Klein and Lee37) and the antioxidant component (the outcome)(Reference Moran, Mohn and Hason38). We conducted supplementary analysis excluding individuals taking blood pressure medication in linear regressions for blood pressure, individuals reporting diabetes medication excluded from linear regressions with fasting plasma glucose and HbA1c measures, and individuals reporting blood pressure or kidney disease medication excluded from linear regressions with an ACR measure.
All analyses were conducted using the statistical software package SPSS version 25.
Results
A total of 324 (45·5 % men) study participants with a mean age of 35·5 (range 15–75) years, representative of the population in the entire community, had a complete profile for cardiometabolic risk markers and plasma carotenoids and vitamin levels.
The study population was representative of the population from the house-to-house census in relation to distribution of sex. A higher proportion of men in the age groups 45–54 and 55+ years participated, and among women a higher proportion of women participated in the age groups 35–44, 45–54 and > 55 years (online supplementary table).
Diet intake in the study population
Men and women had different levels of plasma carotenoids and vitamins (P < 0·05). On average, men had higher geometric mean levels of lycopene (267·0 nM v. 227·0 nM, P = 0·02) and retinol (1·7 µM v. 1·4, P < 0·01) than women, whereas women had higher geometric mean levels of β-cryptoxanthin (37·9 nM for men v. 50·1 nM for women, P < 0·01) and β-carotene (115·9 nM men and 157·1 nM women, P < 0·01) (Table 1).
Table 1 Diet quality, cardiometabolic risk markers and self-reported characteristics in a remote Australian Aboriginal community (n 324)
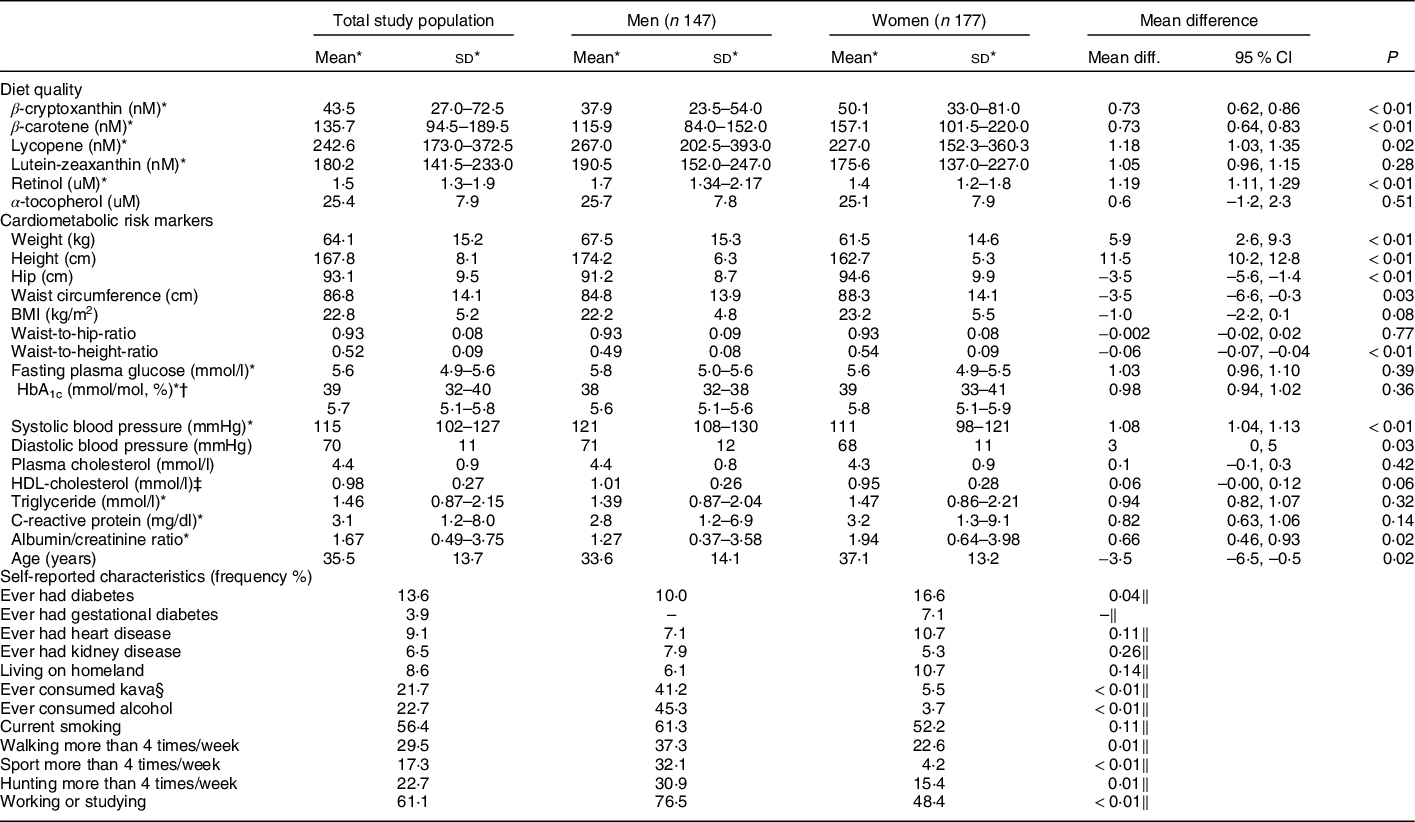
* Back transformed log variables: geometric mean (25th–75th percentile), geometric mean differences and 95 % CI.
† Glycated Hb.
‡ HDL-cholesterol.
§ Kava (Piper methysticum Forst. f.) is a traditionally used depressant from the kava plant root(Reference Cawte68,Reference Clough, Rowley and O’Dea69) .
‖ χ 2 test.
Cardiometabolic risk of the study population
As presented in Tables 1 and 2, the descriptive analyses revealed a difference in the cardiometabolic risk profile for men and women. In the total study, population geometric mean of C-reactive protein was 3·1 mg/dl, 25th and 75th percentile 1·2–8·0 mg/dl, whereas 46·9 % men and 57·7 % women had high levels of hs-CRP ≥ 3 mg/dl.
Table 2 Cardiometabolic risk profile of remote Australian Aboriginal people (n 324) for the total study population and by sex*

* Cardiometabolic risk markers are according to international standards; however, for BMI(Reference Brimblecombe, Mackerras and Garnggulkpuy27,Reference Daniel, Rowley and McDermott70,Reference Daniel, Paquet and Kelly71) and HDL-cholesterol, waist-to-hip-ratio population-specific risk markers are used(31,Reference Maple-Brown, Brimblecombe and Connelly32) .
† Glycated Hb.
‡ HDL-cholesterol.
A higher proportion of women had high BMI levels according to the population-specific cut-off BMI ≥ 22 kg/m2(Reference Daniel, Rowley and McDermott33) compared with men (59·1 % v. 48·6 %), a higher proportion with low levels of HDL-cholesterol (<1·0 mmol/l) (66·5 % v. 53·7 %) and women had higher means of anthropometric measurements (hip, BMI, waist-to-height) and ACR compared with men (P < 0·05), whereas a higher proportion of men had high blood pressure (≥ 120 and/or 80 mmHg) and higher means of systolic and diastolic blood pressure (P < 0·05).
A higher proportion of men reported to be physically active by participating in sport (32 %), walking (37 %) and hunting (31 %) compared with women, respectively, sport (23 %), walking (5 %) and hunting (15 %), P < 0·01. Moreover, a higher proportion of men also reported to ever have consumed kava (41 % v. 6 % women) and alcohol (45 % v. 4 % women), P < 0·01 (Table 1).
The proportion of individuals reporting to take medication for diabetes, blood pressure, heart disease or kidney disease was between 0·3 and 2·2 %.
Antioxidant component levels were higher among those with higher self-reported vegetable intake (P < 0·01), higher among those with higher self-reported fruit intake (P = 0·05) and lower among current smokers (P = 0·06) (Table 3).
Table 3 Diet quality component by self-reported diet intake and smoking status from a remote Australian Aboriginal community (n 324)

Linear regression models revealed an inverse association (β = –0·01, 95 % CI –0·02, < –0·01, P < 0·01) between the antioxidant component and hs-CRP after adjusting for possible clinical and socio-behavioural confounders: age, sex, smoking total cholesterol. The antioxidant component was not statistically significantly associated with the other cardiometabolic risk markers after full adjustment (Table 4). Supplementary analysis also revealed an inverse association (β = –0·25, 95 % CI –0·48, –0·01, P < 0·04) between hs-CRP and the antioxidant component, when conducting the linear regressions without individuals with self-reported chronic cardiometabolic diseases including diabetes, kidney disease, heart disease or gestational diabetes (see online supplementary material, Supplemental Table 7). When excluding individuals with self-reported chronic cardiometabolic diseases, associations between the ACR and the antioxidant components were also inversely associated (β = –0·26, 95 % CI –0·49, –0·03), online supplementary material, Supplemental Table 7.
Table 4 Multiple linear regression antioxidant component (β-carotene, β-cryptoxanthin, lycopene, lutein-zeaxanthin, α-tocopherol, retinol) and cardiometabolic risk markers for the total study population (n 324) of Aboriginal people in remote Australia

* Model 1: unadjusted model 2: adjusted for sex, age, total cholesterol and current smoking.
† Original variables were not normally distributed and were therefore transformed to lg10 values, in this model back-transformed values.
Discussion
In the current study, we examined cross-sectional data from a community in the remote Northern Territory of Australia and have described an overall high risk cardiometabolic profile with low mean levels of plasma antioxidants, similarly low to the low levels reported from a study in an Aboriginal community in Western Australia and below those observed in populations with a lower risk of cardiovascular mortality(Reference Rowley, Su and Cincotta26,Reference Vogel, Contois and Tucker39) .
Antioxidants as a marker for diet quality
Consistent with previous studies(Reference Coyne, Ibiebele and McNaughton18–Reference Souverein, de Vries and Freese20), we found higher antioxidant component levels among those with higher self-reported intakes of fruit and vegetables and lower antioxidant levels among current smokers reflecting concordance between diet and behaviour questionnaire items and plasma levels of carotenoids. Previous studies have reported plasma carotenoids as useful biomarkers of total dietary intake of fruit and vegetables(Reference Coyne, Ibiebele and McNaughton18,Reference Voutilainen, Nurmi and Mursu40) , which is also indicated in the present study. This signifies that high intake of fruit and vegetables is associated with higher antioxidant levels across different communities and populations.
Another factor which influences the levels of antioxidants is the intake of traditional plant foods. A study found higher diet quality among individuals consuming traditional foods compared with individuals not consuming traditional foods among first nations people in Canada(Reference Sheehy, Kolahdooz and Schaefer41). In our study, we found higher mean of the antioxidants component among individuals reporting to consume bushfoods most or every day, although not significant. Native edible plant species in Australia are diverse, extensive and generally nutrient dense. Yunupingu et al recorded 113 plant species used as food or in food preparation among the Rirratjingu Peoples of Arnhem Land. Terminalia carpentariae (Mamanbu to the Rirratjingu Peoples), for example, has very high vitamin C levels of 1141 mg/100 g(Reference Yunupingu, Mrika and Marika42).
Plasma antioxidants and C-reactive protein
We found an association between concentrations of hs-CRP and the antioxidant component, which may suggest that high antioxidant levels may have a protective role on inflammation by the mechanism of carotenoids fighting the increased levels of reactive oxygen species due to their antioxidant role or protecting against oxidation, both which are associated with cardiometabolic risk and higher inflammation indicated by higher levels of hs-CRP(Reference Roberts and Sindhu43,Reference Block, Dietrich and Norkus44) . Cross-sectional study design however limits interpretation on temporality and causation. An alternate mechanism may be that higher inflammation results in less antioxidants in the blood, or higher antioxidant levels, as markers of higher fruit and vegetable consumption, indicate a healthier diet and lifestyle overall.
Another mechanism which may explain the association with hs-CRP is the anti-inflammatory effects of vitamin E by direct anti-inflammatory and antioxidative pathways(Reference Schwab, Zierer and Schneider45). A study from Germany found a dose–response association between high intake of vitamin E and lower hs-CRP levels(Reference Schwab, Zierer and Schneider45).
The inverse association we found between the antioxidant component and hs-CRP is consistent with previous findings from an Aboriginal community from Western Australia(Reference Rowley, Walker and Cohen24), a large national study NHANES III from the USA(Reference Ford, Liu and Mannino46) and a longitudinal study the YALTA-sub-cohort of CARDIA from the USA(Reference Hozawa, Jacobs and Steffes47), all reporting a negative association between plasma antioxidants and CRP. When excluding individuals with self-reported diagnosis of cardiometabolic diseases, we still found an inverse association between antioxidants and hs-CRP, including an inverse association with antioxidants and ACR (a measure of kidney glomerular function)(48). This is consistent with previous studies among Australian Aboriginal populations reporting inverse associations between diet-derived carotenoid antioxidants and lower levels of antioxidants and microalbuminuria(Reference Rowley, O’Dea and Su23) and higher prevalence of chronic diseases(Reference Luke, Ritte and O’Dea25).
Individuals with self-reported chronic diseases already have oxidative stress, which may be associated with lower levels of antioxidants. However, having a chronic disease may be associated with greater awareness of eating fruit and vegetables and thus higher levels of antioxidants.
Pro-inflammatory processes, as marked by hs-CRP levels, are now considered to be causal in the development of heart disease(Reference Aday and Ridker49), and an important contributor to the underlying aetiology of chronic diseases such as metabolic syndrome(Reference Mazidi, Toth and Banach50), diabetes(Reference Dehghan, van Hoek and Sijbrands51) and renal disease(Reference Barr, Barzi and Hughes52).
Importantly, elevated hs-CRP levels appear to be a more stable risk factor than other CVD risk factors as reported previously by a study that used the same dataset as that of our study(Reference Shemesh, Rowley and Jenkins7). Directionality and causal associations are difficult to extrapolate from our cross-sectional findings; however, a small intervention study improving dietary intake of fruits and vegetables among healthy, non-smoking men significantly reduced hs-CRP without effects on other immunologic function markers(Reference Watzl, Kulling and Moseneder53) suggesting a direct and protective relationship between fruit and vegetable intake and hs-CRP. Over half of the sample in our study population had hs-CRP > 3·0 mg/dl, which together with a high proportion of overweight (according to the population-specific cut-off BMI ≥ 22 kg/m2) and adverse cardiometabolic traits would make them at high risk for CVD, so improvements to diet quality via increased antioxidant intake could have positive effects on residual CVD risk.
Other cardiometabolic risk markers
Results of the present study showed a positive but not a statistically significant (P > 0·05) association between plasma antioxidant component and anthropometric risk markers, HbA1c and blood pressure.
The levels of plasma carotenoids and vitamins in the present study are similar to the low levels reported from a study in an Aboriginal community in Western Australia and below those observed within general non-Indigenous and Aboriginal populations with a lower risk of cardiovascular mortality(Reference Rowley, Su and Cincotta26,Reference Vogel, Contois and Tucker39) . The levels of antioxidants in the present population may be too low overall to detect a difference between antioxidant levels on the anthropometric risk markers, HbA1c and blood pressure, despite an observed influence on other pathophysiologic processes, for example, inflammation, as reported in this article. This may explain inconsistencies with other studies, which found a protective effect of carotenoids and antioxidants on cardiometabolic risk factors other than hs-CRP(Reference Rowley, O’Dea and Su23,Reference Rowley, Walker and Cohen24,Reference Voutilainen, Nurmi and Mursu40) . A 10-year follow-up study from Aboriginal populations from Central Australia reported high levels of plasma antioxidants at baseline associated with lower incidence of diabetes, hypertension and CVD at follow-up(Reference Luke, Ritte and O’Dea25). This indicates a likely protective role of plasma antioxidants on the development of cardiometabolic diseases among Aboriginal populations in rural and remote settings, and the potential for a higher quality diet as a target for improving health outcomes among this higher-risk group.
Additionally, the low levels of plasma carotenoids might not reflect other protective nutrients in the diet (such as n-3 fatty acids) not measured in our study that may be acquired through consumption of traditional foods such as seafood which are also associated with anthropometric and chronic disease risk markers(Reference O’Dea54). Factors which may influence the low levels of antioxidants found in this study are the cost, availability and ability to home-prepare and store store-bought fruit and vegetables(Reference Brimblecombe, Ferguson and Barzi55). A study in Western Australia revealed that the areas of the greatest geographic and socio-economic disadvantage had the lowest quality of selected fruits and vegetables as well as the highest prices(Reference Pollard, Landrigan and Ellies56).
Strength and imitations of the study
Strengths of this sub-study include that the study it drew data from was initiated and conducted in partnership with the community. The employment and involvement of local people and researchers to determine study processes and assist with data collection including administration of the health behaviour survey in local language helped achieve culturally safe processes where community members felt safe to participate. The partnership with the community was also a strength in relation to recruitment of study participants. Local community members went door to door, to inform about the community-wide health promotion programme in local language and its potential benefits. This resulted in a high participation across the community. The partnership with the community enabled the community to use the study findings to inform community-initiated and led health promotion activities(Reference Cargo, Marks and Brimblecombe57).
Another strength includes the use of plasma antioxidant levels, carotenoids and vitamins, as an objective indicator of diet quality from fruit, vegetables, fish, wild meats and wholegrain cereals not dependent on self-reported intake of fruit and vegetables(Reference Nybacka, Lindroos and Wirfält21,Reference Fallaize, Livingstone and Celis-Morales22) . As plasma antioxidant, plasma carotenoids and vitamins can be influenced by factors such as sex and lifestyle (e.g., smoking)(Reference Jansen and Ruskovska58) we also attempted to control for these in the regression analyses(Reference Hozawa, Jacobs and Steffes47).
We acknowledge several limitations of the study. Most importantly, the current results are based on a cross-sectional study and thus cannot identify cause-and-effect relationships. We have not accounted for other factors that may also influence hs-CRP levels, such as infections. Another important factor previously reported to influence levels of antioxidants is socio-economic status(Reference Hodge, Cunningham and Maple-Brown59) and the role of energy cost influencing food choices(Reference Brimblecombe and O’Dea15,Reference Drewnowski and Specter60,Reference Brimblecombe, Maypilama and Colles61) , which likely could have an influence on our results. However, data regarding socio-economic status and other cultural factors were collected in a manner acceptable to the community and by request not included in the questionnaire. Data on these factors could be useful in further investigating the association with plasma antioxidants and the cardiometabolic risk markers as they may show that those reporting a higher socio-economic status may be those with higher carotenoid levels due to ability to buy and home-prepare foods high in antioxidants. Additionally, diagnoses of cardiometabolic diseases and medication were only collected as self-reported information. We examined ten cardiometabolic risk markers separately so multiple statistical comparisons were made, thereby increasing the chance of observing statistically significant results. We did however plan to examine all risk markers ahead of conducting the analysis and a 5 % significance level applies to each test considered individually.
Implications for nutrition research and health practice
With the present findings of the association between low plasma antioxidants as markers of low diet quality and higher levels of hs-CRP, the current study provides further evidence of the importance of improving the diet quality. We also found that self-reported high intake of fruit and vegetables was associated with higher levels of the antioxidant component in this population consistent with previous findings from other communities, indicating the benefits of policies aiming to increase the intake of fruit and vegetables, which subsequently is associated with increasing the levels of antioxidants in this population as well as of other populations. Increasing the diet quality especially in remote Aboriginal communities requires an understanding of the underlying multiple facet factors, such as structural factors (i.e., food insecurity including food affordability, access, supply and storage issues), and knowledge that local(Reference Strate, Brimblecombe and Maple-Brown6,Reference Brimblecombe and O’Dea15,Reference Cargo, Marks and Brimblecombe57,Reference Ferguson, Brown and Georga62) initiatives have proven useful and effective in maintaining a healthy BMI but require continued resources to maintain(Reference Strate, Brimblecombe and Maple-Brown6,Reference Ferguson, O’Dea and Altman63) . This highlights the need for future plasma antioxidant and nutrition research with Aboriginal communities in remote Australia to focus more on increasing the diet quality, with higher intake of fruit, vegetables (including traditional plant foods – leaves, tubers, seeds, nuts, fruit, stems, etc.), wild meats, fish and seafood through improved availability and affordability, which could include store-based and local food production and procurement strategies and policies, community-wide promotion of healthy foods and healthy food pricing policies. On feedback of these study findings to community organisations, community members requested that information on the protective anti-inflammatory role of antioxidants be made available to them in the form of a resource so they could communicate this information more widely in their community.
With respect to diet, it is important to recognise that fruit and vegetable consumption is just one indicator of diet quality. Focus is also needed on enabling sustained access to wild meats, fish, seafood, plant foods and improved wholegrain cereal intake. Wild plant and animal foods, especially organ meats, are rich sources of antioxidants and for millennia provided nourishment and protected from cardiometabolic diseases among Aboriginal and Torres Strait Islander nations across the continent(Reference O’Dea, Naughton and Sinclair64). Other factors outside of the quantitative analytical approach in the present study, such as the social collectivist context of lived Aboriginal experience(Reference Hogg, Roe and Mills65), the value of traditional food, communal eating and sharing of food as well as vital cultural factors such as connection to Country(Reference Burgess, Johnston and Bowman66,Reference Jones, Thurber and Wright67) , and political determinants such as self-determination, autonomy and control of funding and Aboriginal leadership in health promotion, also contribute to cardiometabolic well-being for Aboriginal peoples.
Conclusions
The current study shows that higher plasma carotenoid levels within this study population of Aboriginal people in very remote northern Australia are associated with lower levels of hs-CRP, indicating a lower risk of CVD. However, only longitudinal data will be able to provide insights as to if, and how, these associations modify risk of cardiometabolic disease within this Aboriginal community. Plant foods, fish, seafoods and wild meats, as important sources of antioxidants, are critical to the health and well-being of this population with disproportionately high chronic disease burden; policies to address availability and affordability to support their intake are urgently needed.
Acknowledgements
Acknowledgements: The current study would not have been possible without the irreplaceable contribution of Yalu’Marnggithinyaraw and the community health service. We also acknowledge Elaine Maypilama and Dorothy Yunggirrnya who assisted with feedback of this research to the community. Financial support: The current study was funded by the National Health and Medical Research Council of Australia (#124319). Conflict of interest: The authors declare that there are no conflicts of interest. Authorship: L.K. and J.G.L. took lead in writing the manuscript and statistical analysis. L.K., J.G.L., K.O., D.L.C and J.K.B. contributed to formulating the research question, designing the study, analysing the data and writing the article. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the combined Human Research Ethics Committee of the Menzies School of Health Research and the Northern Territory Department of Health and Community Services. Written informed consent was obtained from all participants.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020004899







